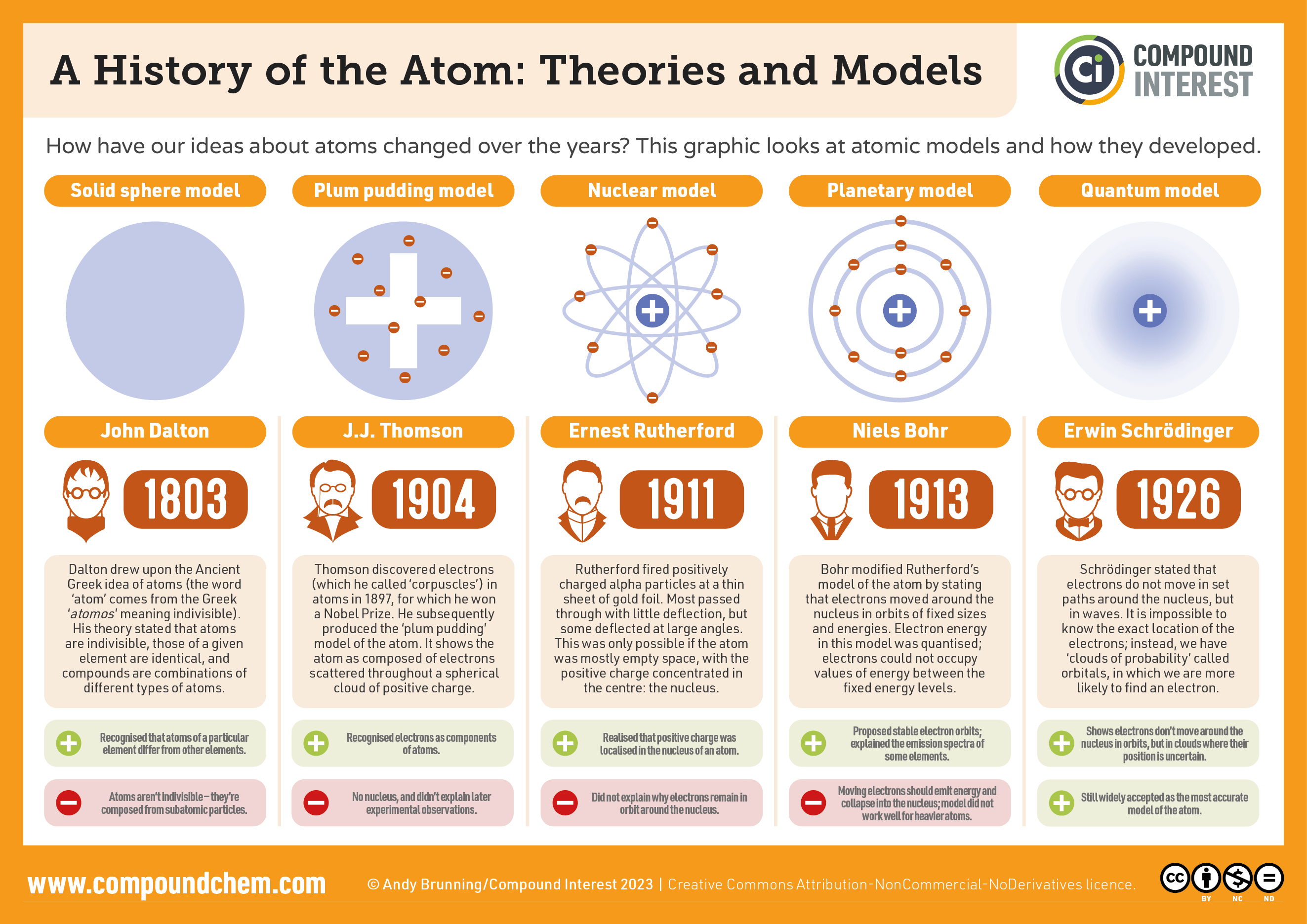
- John Dalton:
- Atomic theory of matter
-
- Elements are composed of extremely small, indivisible particles called atoms.
-
- All Atoms of given element are identical having same size, mass and chemical properties. Atoms of different elements have different size, mass and chemical properties.
-
- Atoms are not created or destroyed during chemical reaction.
-
- A chemical reaction involves only separation, combination. Or rearrangement of atoms.
-
- Compounds are formed when atoms of more than one element combine in a specific ratio.
- TLDR John Dalton proposed everything is made up of extremely small particles called atoms.
- why bad:
- JJ Thomson
- discovered the electron
