
Consider checking out the Edward ATAR 12 Redox Seminar Masterclass for a shortened version of the entire Redox course.
Redox Introduction
- Reduction & Oxidation
Read question carefully depending if it asks you to explain in reduction or oxidation.
- Reduction
- gain of electrons
- Oxidation
- loss of electrons.
- due to conservation of matter, reduction and oxidation is the exchange of electrons, and happens simultaneously.
- OILRIG
- oxidation is loss, reduction is gain
- example:
- oxidisation: (oxidation half equation)
- reduction: (reduction half equation)
- balance:
- combine: (full redox equation)
- Consider the oxidation number
- Cl2: 0
- 2Na: 0
- 2NaCl: +1 -1
- increase in oxidation number: oxidation
- decrease in oxidation number: reduction
common types of redox reactions
- metal metal ion displacement
- metal transfers ions
- halogen halide ion displacement
- electrons transferred to a halogen, halides to less reactive halogen
- combustion
- corrosion
Oxidation Numbers
- increase in oxidation number: oxidation
- decrease in oxidation number: reduction
Tip
Oxidation number (oxidation state): A number assigned to the atom to show how many electrons lost, gained or shared unequally (to form a bond).
- Oxidation number of Na: 0
- Oxidation number of Cl2: 0
- Oxidation number of Na in NaCl: +1
- Oxidation number of Cl in NaCl: -1
- Oxidation number of H in HCl: +1
- Oxidation number of Cl in HCl: -1
- Oxidation number of H in H2O: +1
- Oxidation number of O in H2O: -2
- Oxidation number of F in F2O: -1
- Oxidation number of O in F2O: +2
- because F is more electronegative than oxygen
| elements with fixed oxidation states in their compounds | oxidation state |
|---|---|
| hydrogen (except in metal hydrides - metal combined with hydrogen & fluorine) | +1 (-1) |
| oxygen (except in peroxides) | -2 (-1) |
| group I elements | +1 |
| group II elements | +2 |
| aluminium | +3 |
| halide | -1 |
- for uncombined elements, the oxidation state is zero
- for atoms in elements (when element combined to itself), the oxidation state is zero.
- for simple ions, the oxidation state is the charge of the ion.
- e.g. +1 and -1
- electronegativity: ability to attract bonding pair of electrons hence something peroxide (???)
- halides: ions of halogens
| halogens | halides |
|---|---|
- if theres one other electron added to it it is not a halide!
by convention put oxidation number under element, and the total above element. this is a common mistake !!
Warning
BE SPECIFIC AND BE PARTICULAR. DO NOT BE VAGUE. EXPLAIN WELL AND LOGICALLY. E.G. “IT” NO GOOD 🙅
- in oxidation, the thing being oxidised is called the reducing agent!
- vice versa
- H2O something something idk
- decrease in oxidation number: reducing: oxidation agent!
- An oxidising agent accepts electrons and is itself reduced.
- increase in oxidation number: oxidising: reducing agent!
- A reducing agent donates electrons and is itself oxidised.
- すごい
Writing Balanced Equations
Practice
Copper (I) Oxide
- Cu2O Manganese (IV) Oxide
- MnO2 Iron (III) Sulphate
- Fe2(SO4)3
New Types of Acids:
- Chloric (I) acid
- Cl is +1 because O is more electronegative :)
- Chloric (V) acid
Combining Half Equations
- Combine it to write balanced equations!
- Reactants go together with reactants products go together with reactants.
- cancel everything out.
Balancing Redox Equations in Acidic Conditions
- Assign Oxidation Numbers
- Write the skeletons of the oxidation and reduction half-equations.
- Balance all elements other than H and O
- Balance the oxygen atoms by adding H2O (where needed)
- Balance the hydrogen atoms by adding H+ ions (where needed)
- Balance the charge by adding electrons, e-
- If the number of electrons lost in the oxidation half-reaction is not equal to the number of electrons gained in the reduction half-reaction, multiply one or both of the half-reactions by a number that will make the number of electrons gained equal to the number of electrons lost.
- Add the 2 half-reactions as if they were mathematical equations. The electrons will always cancel If the same formulas are found on opposite sides of the half-reactions, you can cancel them. If the same formulas are founded on the same side of both half-reactions, combine them.
- Check to make sure the atoms and the charges balance.
disproportionation
- 1 substance is simultaneously oxidised and reduced
- has the general equation:
displacement reaction of halogens
- describing appearance of halogens
- ms pilling demonstration
| halogens | halides |
|---|---|
| Cl2 + 2e | 2Cl- |
| Br2 + 2e | 2Br- |
| I2 + 2e | 2I- |
| (oxidising agents) | reducing agent |
- if a substance is coloured, it is not due the halides. hence only halogens and colours are listed on page 5 of data booklet.
- halides could be oxidised to halogens
- series of chemical reactions, observe outcomes, and then make predictions
- Cl2 + Br- -> Cl- + Br2
- ox:
- red:
- yellow solution added to orange solution forming orange solution (?)
- cell potential calculations: positive, therefore feasible.
- ox:
- red:
- no visible reaction
- no visible reaction
- no visible reaction
Electrochemical Cells
Galvanic Cells & Daniel Cells
- what is galvanic cell:
- a galvanic cell uses two half-reactions in separate containers and makes use of the spontaneous nature of the reactions to generate an electric current. potential chemical energy converted to potential energy.
- the transfer of electrons in the redox reactions is harnessed through physical separation of the two half-equations.
daniel cells
- this is a daniel cell
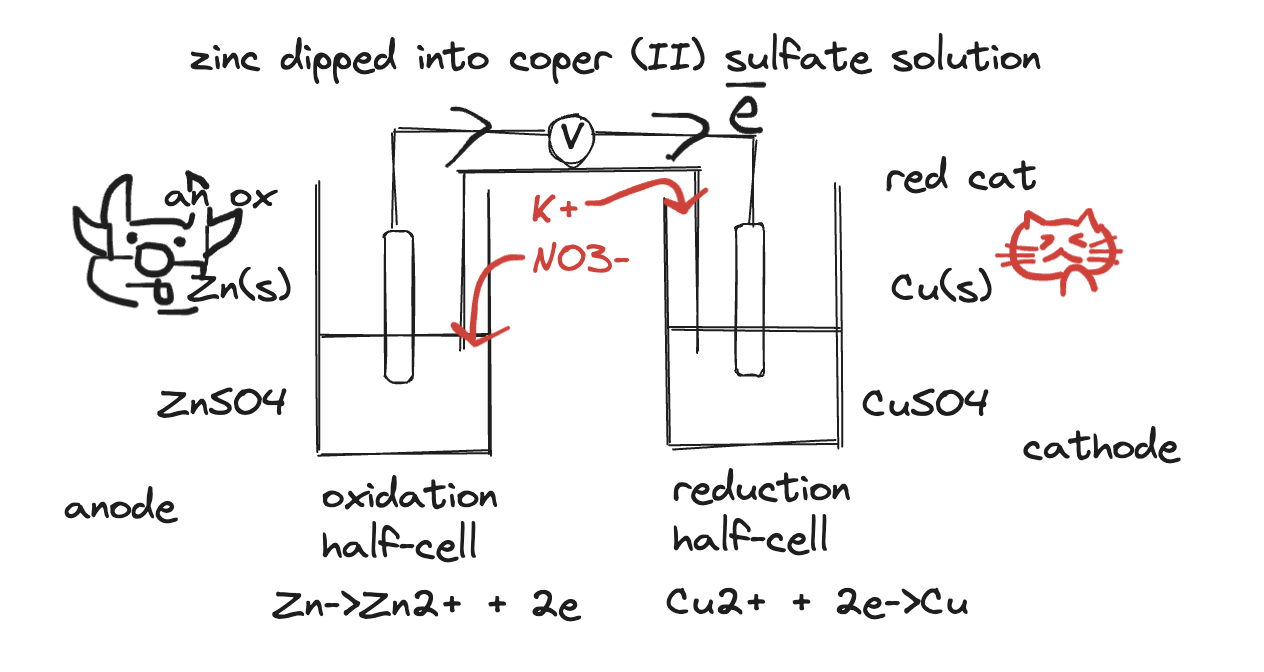
- purpose of salt bridge: joins the two half cells. complete the circuit and ___ ms pilling will give booklet with better description. movement of ions in salt bridge allows flow of charge through the cell and complete the circuit.
- purpose of high-resistance voltmeter: reduce the current because you don’t want dramatic drop in voltage.
- zinc is more reactive, so oxidises and loses electrons that flow blah blah blah blah
- the solid thingy is usually graphite or platinum.
Anode and Cathode
- An ox & Red cat
- Anode is negative, Cathode is positive (in galvanic cells only - in electrolytic cells anode is positive and cathode is negative!)
Feasibility of a Reaction
- A reaction is thermodynamically feasible if calculated is positive.
- choose to change sign or not -> if it is changed it is - oxidised instead.
- same outcome lol - depends on question on what you need to do, mathematically they are the same formula.
- +- oxidised depending on if sign is changed.
Sacrificial Anode
- sacrificial anode: strong reducing agent (more reactive) metal that will be oxidised preferentially attached to iron (or other metals) to protect it (from oxidising)
Ms Pilling on Self Study
its like any other sport - you dont do training, you dont exercise, you dont get the prize
Cathodic protection
- ICCP, Impressed Current Cathodic Protection systems are used to provide cathodic protection for pipelines, ship hulls, offshore production platforms, water treatment equipment etc.
- principal advantage of ICCP is greater output capacity compared to sacrificial anode systems - therefore when protection is desired for large, poorly coated and bare structures ICCP is often choice.
- it requires external DC power source that is energised by standard AC current.
- insert cathodic protection diagram
- protected structure
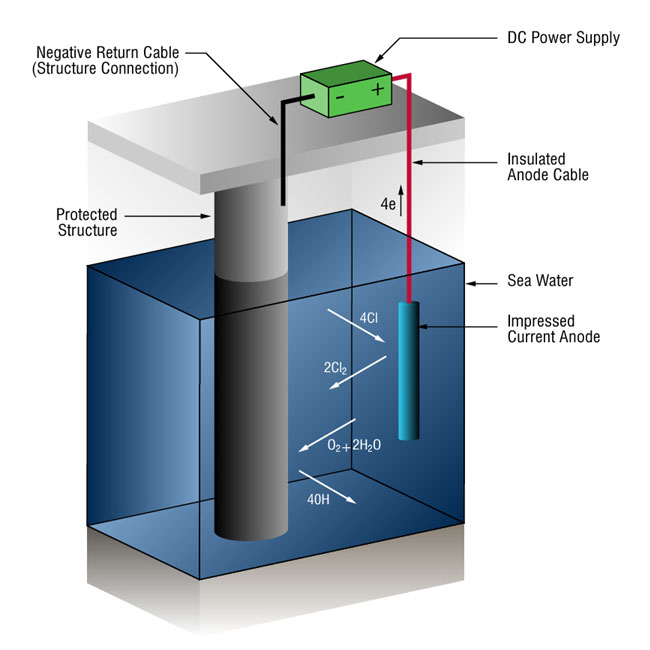
- sea water
- many different ions: sodium ion
- sodium ion -> metal
- too much energy, not provided
- many different ions: sodium ion
- enough energy to turn chloride into chlorine! (oxidised) on anode
- moving to cathode, and chlorine is reduced to chloride
- if theres enough current, could happen (?)
- moving to cathode, and chlorine is reduced to chloride
sacrificial anode CP method vs impressed current CP method
- sacrific
- no external source of power
- spontaneous reactions
- oxidised more readily than iron
- cathodic protection
- impressed current
- metal and anode, less reactive, acting as cathode.
- non spontaneous reaction.
Corrosion
- oxidisation of a metal
- usually occurs in the presence of oxygen and water vapour in the air to form metal oxide.
dry corrosion
- dry corrosion/oxidation occurs when oxygens or other reactive gases in air reacts with exposed metal without the presence of a liquid. This causes an oxide layer to form over the metal, which damages its surface properties.
- oxide layer called rust.
- dry corrosion requires contact between air and metal, so oxide layer forms eventually stops (as it no longer is in contact because the layer prevents it)
- this stopping is known as passivation.
- oxide layer is porous in some cases, and thus corrosion can continue deep into the material.
- known as active corrosion.
- porous is basically having holes
- dry corrosion is sensitive to temperature: reacts faster under application of heat (no way rate of reaction???!)
3 types of dry corrosion
oxidative corrosion
- point of contact between metal and o2 gas: o2 gas is reduce and oxidises metal
- this forms metal oxide.
- metal oxide may be stable: adhere onto non-corroded metal; this prevents more contact between o2 and metal; therefore no more corrosion.
- the metal oxide may be stable: but NOT adhere onto the non-corroded metal (i.e. might flake away): it then fails to prevent direct contact between oxygen gas and metal, and metal corrosion continues
liquid metal corrosion
- occurs when liquid metal is allowed to flow over solid metal at high temperature.
- one example is flow of sodium metal (used as coolant) over cadmium. cadmium is corroded and sodium ions are reduced.
corrosion by other gases
Fuel cells
- h2 and o2 come to make h2o
- this is foundation of fuel cells.
- spontaneous reaction, produce lots of energy.
- anode is where hydrogen are
- cathode has oxygen
- electrons travels from anode to cathode.
- electrolytes block.
- catalyst helps hydrogen gas to split apart
advantages
- uses external fuel source, so not reliant on limited store capacity
- high efficiency
- emission-free; zero emissions, only water vapour
- high energy density: a lot of energy in small volume
- renewable: hydrogen can be produced from renewable energy sources, such as wind, solar, hydropower
disadvantages
- high cost to produce a fuel cell
- production/transportation/distribution/storage problems with hydrogen
- developing technology ? i think you have to expand on this. check practice tests later and come back.
applications of redox: primary/secondary/fuel cells blah bla
cells and batteries
- redox: transfer of electrons between 2 substances. separate galvanic cells and the movement of electrons between external circuit to generate electricity.
- electrochemical cell is a store of chemical energy in closed system: closed regards with chemical substance, all reactants and products contained within the battery, but not closed with respect to energy transfer (physics concept woohoo)
- split into primary and secondary cells (rechargeable and non-rechargeable respectively)
- secondary cell can be discharged and recharged =D
- size of battery means more chemicals inside which means it can run for a longer amount of time; it does not affect the voltage output.
a battery is more than one cell connected together in a series arrangement
- the electromotive force (voltage) increases when the cell is connected together in series. voltage = sum of voltages of each cell.
- a battery is a closed system which contains high energy reactants and low energy products in a sealed unit.
primary cell
- lots and lots of primary cell but only two we learn =D they all have relatively similar patterns.
- one use wonder! non reversible.
- advantages
- cheap
- don’t discharge unless power is drawn
zinc-carbon dry cell - leclanche cell
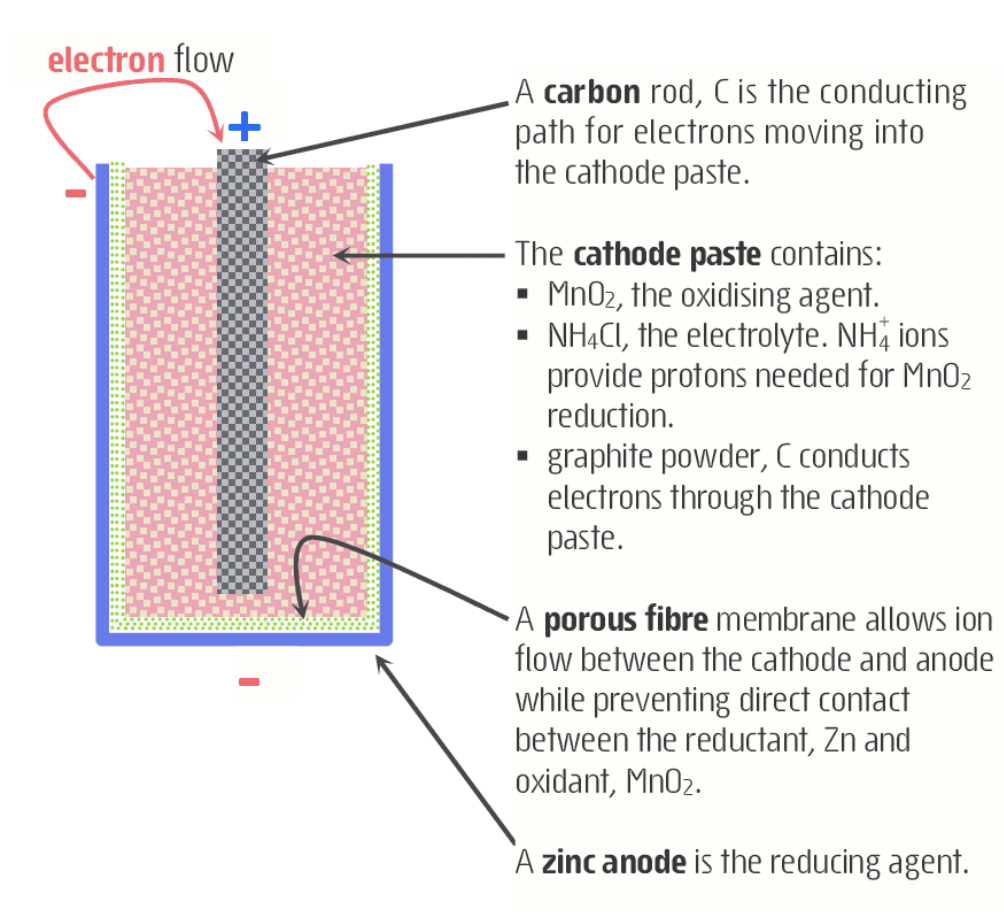
- produces 1.5V
- carbon is inert (non-reactive) and conductive electrode: hence why it is used. if a metal is used, it would react and that wouldn’t be poggers.
- porous fibre is like filter paper fr no cap.
- allows outside to move between the thing and the slightly red paste; outside is zinc.
- excess zinc is used, so it won’t be entirely consumed and the zinc ions wont leak everywhere.
- half reacitons:
- ox
- red:
- full
- disadvantages
- low energy to mass ratio
- advantages
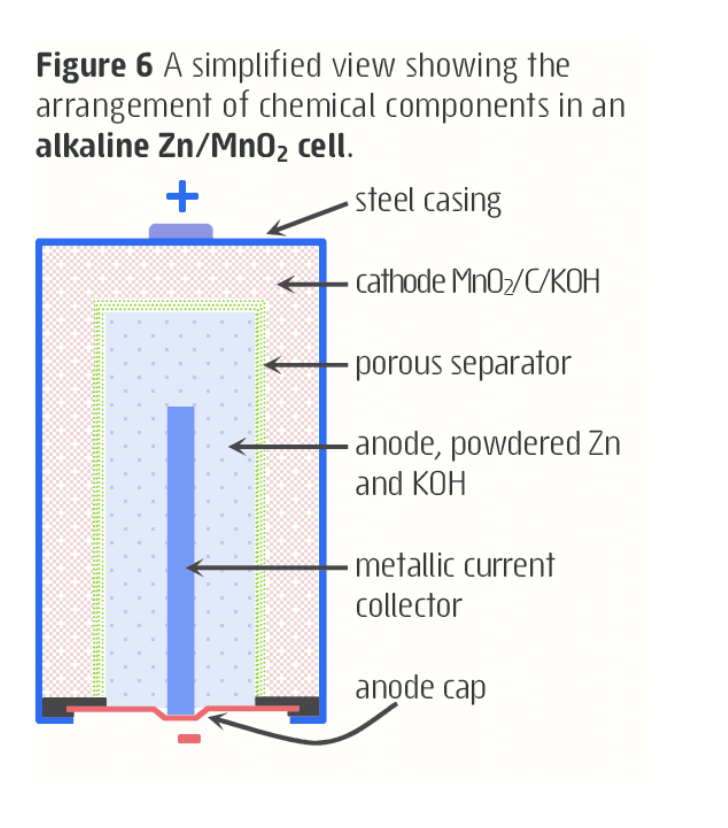
alkaline cell
- uses zinc and mangaese(IV) oxide, but uses potassium hydroxide paste as electrolyte.
- alkaline cells do not need lots of electrolyte, so more space for reactants.
- thus it has higher energy to mass ratio as a result.
secondary cells (rechargeable)
- secondary cells are a massive part of the modern world
- utilises fairly new (with regards to redox topic)
- we make things go backwards (do, undo, redo, undo blah blah blah)
- process that goes both ways :fire: bars
- rechargeable cells got invented
- chemical energy -> discharge spontaneous energy -> electrical energy -> recharge non-spontaneous reaction -> chemical energy
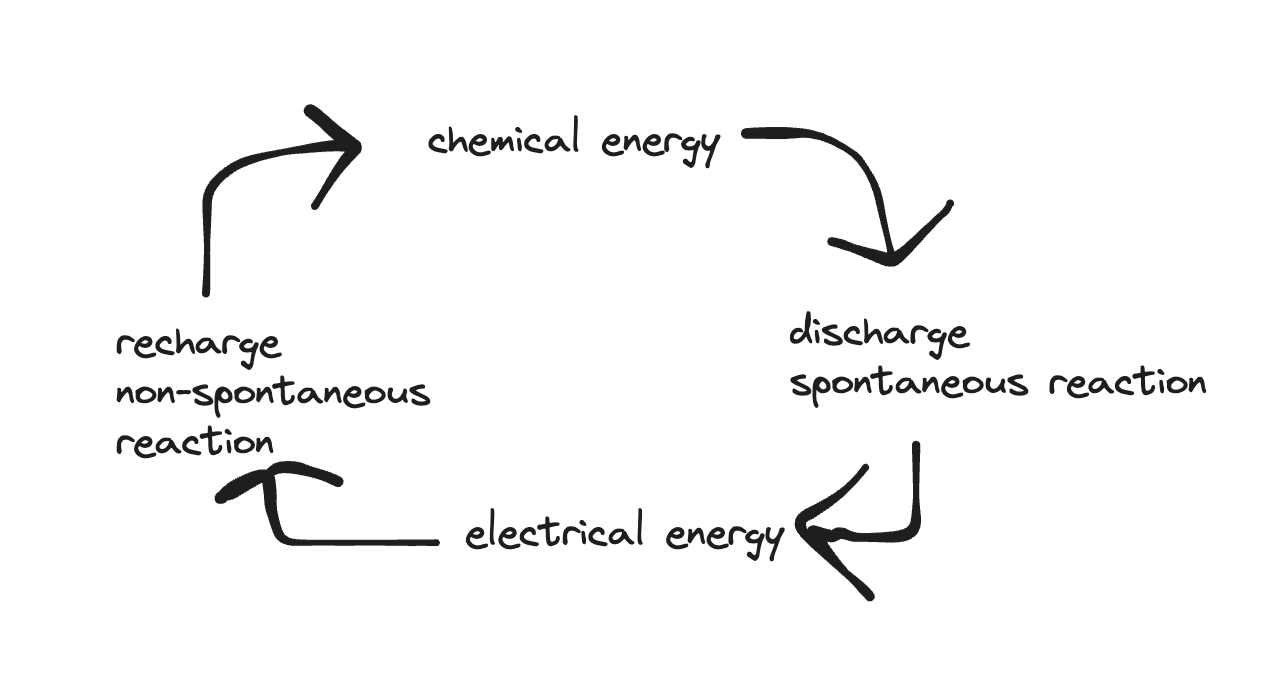
- once discharged, recharged by applying dc voltage (have to mention dc voltage!)
- we do not use reversible symbols in redox equations; discharge with forward arrow or recharge with a forward arrow
nickel metal hydride cells
- dont need to memorise diagram
- nickel metal hydride batteries results in high demand for nickel.
- mysterious rare-earth metals: alloy, store hydrogen as hydride
- recharge reverses reaction; in this case me turn the metal alloy back into hydride, so we do half reaction in opposite direction.
- talk about polarity later
- ms holland will talk about electrolysis first
lead acid battery
- CAR BATTERIES
- NOT TESLA BATTERIES. REGULAR PETROL-DIESEL
- PURPOSE IS TO TURN THE STARTER MOTOR.
- delivers HIGH VOLTAGE and HIGH CURRENT to turn over the motor getting it starting, reducing the mechanical resistance.
- each cell produces 2V, car battery uses 6 cells in series to output 12V
- advantages
- cheap reliable
- very reliable, withstand many discharge-recharge cycles: long lifetime
- output large current
- disadvantages
- low energy density
- safety hazards; corrosive sulphuric acid electrolyte; toxic lead compounds.
- if broke, waterway can say byebye
- environmental concern upon end of life.
- horrible pollutant, lead will troll the environment.
- porous anode, to increase surface area
- cathode made with lead (4) oxide
- all good to go again once recharged.
lithium ion battery
- lithium exists in cathode and in electrolyte.
- cathode made with lithium cobalt oxide
- when battery is fully charged, we shoved lithiums in graphite
- discharge reactions:
- anode (-) Lic6 -> Li+ + c6 + e-
- cathode (+) coO2 + Li+ + e- -> LiCoO2
- redox: LiC6 + CoO2 -> C6 + LiCoO2
- different materials for two different electrodes results highly energetic favourable electrodes with different materials.
- recharged anode -> cathode, cathode -> anode.
- fire risk
- banned on flights due to the fire risk they pose
-
- short-circuiting
- porous separator keeps battery electrodes apart; charging for long periods can damage the porous membrane, removing it cause battery to discharge rapidly and generate a lot of heat.
-
- overcharging
- when overcharged, lithium cobalt oxide releases oxygen. react with flammable electrolyte and also cobalt oxide
-
- electrolyte breakdown
- electrolyte catches fire.
Fuel Cells
- Does not form the definition of electrochemical cell.
- It is not a closed system, but it is instead an open system.
- It is not being used to replace batteries, we use it more often to replace a combustion engine.
- Direct replacement to engine in vehicles.
- Hydrogen Fuel Cell
- Combustion Reaction of Fuel is the only reaction simplified, unless it wants you to state the half equations.
- There are multiple kinds of fuel cells: 3 kinds of Hydrogen Oxygen cells (didn’t catch what she said)
Hydrogen Oxygen Cell
- Electrochemical Energy: Converts between CPE and EE
- Spontaneous reaction: CPE -> EE (galvanic type redox reaction)
- Most common fuel cells
- Ms Holland likes anode on the left=D
- Pretty efficient, but it is a bit inefficient too, so some energy is converted to heat.
- Structure: Electrolyte (3 types of Hydrogen Oxygen Cells)
- Acidic Kind:
- H2SO4 sulphuric acid
- good for labs
- not good for industries
- phosphoric acid
- good for industries
- H2SO4 sulphuric acid
- Alkaline:
- KOH (potassium hydroxide)
- both lab and industries
- KOH (potassium hydroxide)
- PEM (Proton Exchange Membrane)
- minor intricate differences between all 3
- Acidic Kind:
- Porous Carbon
- Tiny Holes
- +Catalyst (sometimes)
- changes depending on version
- Anode, Cathode Compartment
- Electrical Energy comes from the movement of electrons.
- Increase voltage by connecting batteries in series.
- Advantages
- Could replace power stations, main idea is to replace fossil fuel engines and batteries, which are both pollution.
- Only requires Hydrogen and Oxygen, and does not produce Carbon Dioxide or pollutants.
- Last longer than batteries, less polluting to dispose of.
- Disadvantages
- Hydrogen is a gas
- hard to fuel up, high flammable and not poggers.
- more space to store than fossil fuels or batteries
- Explosive when mixed with air
- will explode in contact with air.
- storage is dangerous
- BIGGEST ISSUE: Hydrogen gas requires energy, and its often produced using fossil fuels.
- Hydrogen is a gas
- aqueous acidic hydrogen oxygen fuel cell: has extra thing at anode for depleted fuel and other gases to come out: catalyst provides conductive surface for thing to occur, platinum doesn’t corrode (expensive for jewellery)
- proton exchange membrane works similar to aqueous acidic.
- production of hydrogen
- hydrogen is a very reactive element
- reaction of hydrocarbons with steam
- electrolysis of acidified water
- hydrogen is a very reactive element
- TRANSPORTING HYDROGEN
- liquify under pressure
- very hard tho, you can force it into liquid state with high pressure, but pressure is ridiculously low.
- expensive method of storage
- adsorption
- take place at low pressure and close to room temperature
- however, materials being used for adsorption does not last forever.
- absorption
- absorbed by solid materials, enter spaces in metal alloys lattices, forming hydrides
- alloys deteriorate over time, and need to be replaced regularly
- liquify under pressure
- hydrogen fuel bus
- since transporting is difficult, some vehicles makes hydrogen using hydrogen-rich fuels and then using it with fuel cells.
- direct methanol fuel cells
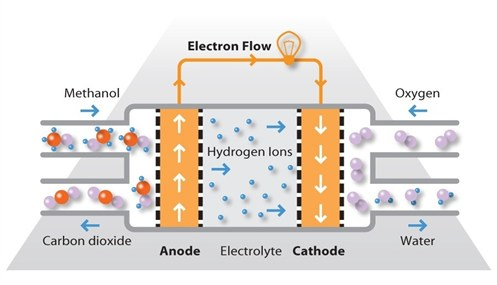
- anode: ch3oh + h2o -> 6h+ +6e- + co2
- cathode: 3/2 o2 + 6h+ + 6e- -> 3h2o
- redox: ch3oh + 3/2 o2 -> co2 + 2h2o
- making ethanol
- less toxic more energy dense alcohol than methanol.
- not ready, as catalysts are not cheap !! very expensive $$ ahhh
- cheaper, more efficient catalysts capable of fully oxidising ethanol are needed.
- pros cons fuel cells
- powerpoint slide on ms holland fuel cells ppt, put the slide here LATER AT SOME POINT ! THANKS!!!!
- breath alcohol tester.
- fuel cell technology used to breath alcohol testers.
Electrolysis of molten compounds
"im dababy" - ms holland
"probably saw a year 8" - ms holland referring to arvin
- non-spontaneous redox reactions
- similarities and differences aqueous and molten
- industrial application electro-refining, electroplating: big in WACE (and probably in pmod as well.)
- watched GCSE Chemistry Part 1
What is electrolysis?
the process of using an external potential difference to supply electrical energy to enable a non-spontaneous reaction to occur.
- electricity to make non-spontaneous redox reactions occur
- many uses
- purifying metals (such as copper)
- plating metals (silver or gold)
- extracting reactive metals (such as aluminium)
- making chlorine, hydrogen (not cheapest way, but byproduct of making chlorine) and sodium hydroxide (how most sodium hydroxide is made)
- electrolyte needs to be liquid: it doesn’t have to be solution, and can be molten instead.
- as long as ions are mobile (free to move), it is O.K.
- carbon electrodes pretty popular in molten aqueous: graphite
- as its pretty inexpensive (? not sure if she said this) and it can withstand pretty high temperature.
- battery pushes electrons and forces in non-spontaneous direction.
- “this gives the theoretical minimum potential difference that must be applied for the reaction to occur”
- in practice, a larger potential difference is required, to overcome energy loss in the system.
electrolysis extracting
- we dig up aluminium oxide, and turn it into pure aluminium
- aluminium is very useful
- aluminium ore (bauxite) has very high melting point.
- the ore is dissolved in compound called cryolite, which lowers the melting point to 700.
- inert carbon lining steel container.
- aluminium gets reduced, sinking to bottom, oxygen oxidises into gas.
- aluminium oxide -> aluminium + oxygen gas
- oxygen produced at the anode will react with the carbon, producing co2, anodes are replaced periodically.
environmental impact
- starting off with aluminium oxide, has to mine it
- mine it is bad for environment when you dig a big ass hole :3
- environmental ecological impact on ecosystems and animals and plants
- energy consumption of diesel and coal and stuff in the process of mining BAD BAD BAD !!
- processing is expensive so we dont do in australia
- so we ship it across the world
- energy consumption from fuel, crude oil, fossil fuels.
- go to china! cheap work yay child labour
- china cheap energy, cheapest coal, brown coal, low energy density and filthy!!! yucky!!!
- aluminium gets made there ^.^
- big cities in china pollutant because brown coal is getting burnt. carbon dioxide hooray!
- aluminium gets shipped back.
- AHH!! scary
- melting bauxite needs energy (comes from brown coal)
- electrolysis requires energy (requires brown coal)
- massive energy demand and massive environmental impact!! =D
- recycling is good, making new aluminium is not poggers for the trees.
- recycle !!! - ms holland
Electrolysis of Solutions
- In theory removing the need to melt, but in practice, adding another compound to melt.
- Aqueous solution is used, allowing the process to take place at lower temperatures.
- However, presence of water affect outcome of some electrolytic reactions.
- Instead of molten compound, you have a solution, and provides a potential difference.
- thought process
- which substances are present in the cell
- which substances can be oxidised
- which substances can be reduced
- which substances are the most energetically favourable at each electrode? (process for least energy overall likely most favourable, use SRP to help)
- which substances are present in higher concentrations? substances present at very low concentrations are less likely to produce bulk of product.
- which substances are present in the cell
- if there is two possible oxidation reactions, and the provided voltage allows both to occur, the two possible reactions happens simultaneously.
- layer of solid copper forms at cathode =D
Electrorefining: Reactive Electrodes
Reactive Electrodes
- you can replace the inert electrodes with reactive electrodes.
- swapping out the electrodes for copper!
- Cu (s) -> Cu (s) if copper is the most reactive thingy. E cell would = 0, and what will happen is that copper will move from the anode to cathode.
- potential difference applied needs to be >= 0
Electrorefining / Electrowinning
- Impure metal is the anode
- Highly pure metal as cathode
- Anodic Slime
- can be very valuable because sometimes gold is there :3
Electroplating
- higher current, higher rate of electroplating
- this can be increased by increasing concentration!
- chromium forms oxide layer that utilises passivation to protect the metal underneath.
- oxygen and cl2 in solution.
- products of sodium chloride solution produces three very useful products
- chlorine
- hydrogen (without use of unsustainable means)
- seawater; financially viable
- chlorine is expected as product but hydrogen and NaOH is a fun surprising result.
- if ionic compound containing mental has negative SRP< electrolysis of solution of compound will usually produce hydrogen rather than the metal.
- if a metal has negative srp - it will struggle to be reduced (?) you get hydrogen instead sometimes
brine electrolysis: chloralkali process
- solution becomes basic and you make sodium hydroxide solution!
- most energetically favourable reactions will occur for reduction/oxidation
- ion only membrane : allows ion through, but not gases! this stops the reactions that is spontaneous for electroplating.
- concentration sodium chloride solution
- dont do competing reactions !!!
- chloride ions being used flow through.
- electrolysis can be used to split h2o into tis elements, hydrogen and oxygen.
- add something to boost its conductivity; simple solution is add sulphuric acid1
- hydroxides -> oxygen water is an option if there are hydroxides
- +1.23 O2 + 4h -> 2h2o
- electrolysis of water
- at the negative electrode:
- 2h+ + 2e- -> h2 (reduction)
- at the positive electrode:
- 4oh- -> 2h2o + o2 + 4e (oxidation)
- at the negative electrode:
CAP THINGS
- check resources of lucarelli 7.6-7.8, 8.6-8.8
- read pearson chapter 10, industrial examples very useful in pearson, more familiar, less likely youd get caught by it questions:
- lucarelli set 11 & set 12 (q16 onwards) HAND IT NEXT WEEK
- stawa sets 22 & 23
- DIRECTLY PREPARE YOU!!!!!!!!!!!!!!!!!!! FOR THE CAP!!!!! DUE WEDNESDAY 21ST!!
- ms holland %
- as you go along, you mark it
- give it a % for the piece of work
- often examiners start short answer with things to shift modes and let you write something immediately.