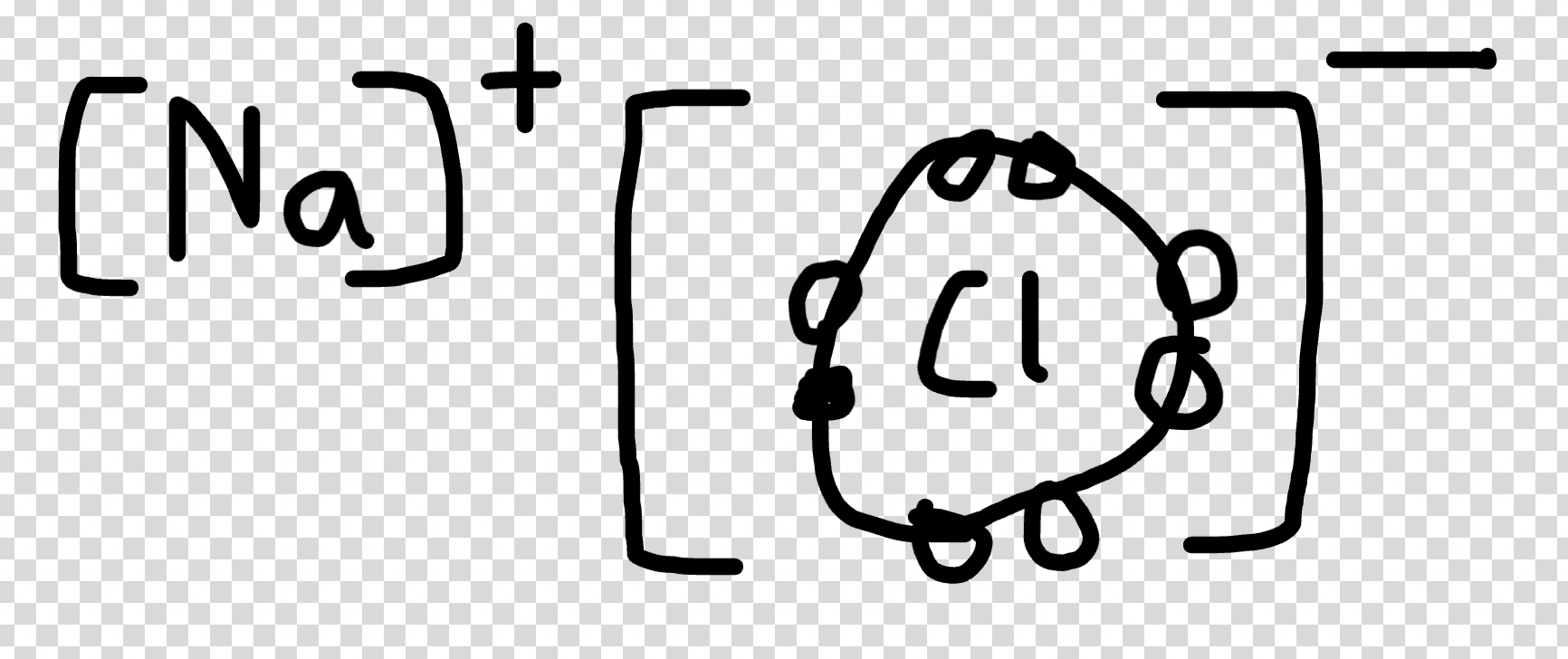different types of bonding
- how to link electronic configuration of elements on the periodic table bonding.
- yesterday talked about metallic bonding.
- alloy is mixture not compound.
- bronze or brass, which is a mixture of different metals.
ionic bonding
electrostatic force of attraction between oppositely charged ions in network.
- metal (not entirely true, for year 11 foundation chemistry it is fine) & non-metal.
- examples:
- NaCl
- MgO
- CaCO3 (metal cation + non-metals anion)
- etc etc etc etc
- to achieve stability of nearest noble gas, for example sodium, will lose an electron
- sodium chloride
- as a result you get sodium chloride compound () in 1:1 compound.
- important to show ratio because it is a molecule and not a network.
- magnesium chloride
- , 1:2
- sodium chloride
- lattice structure
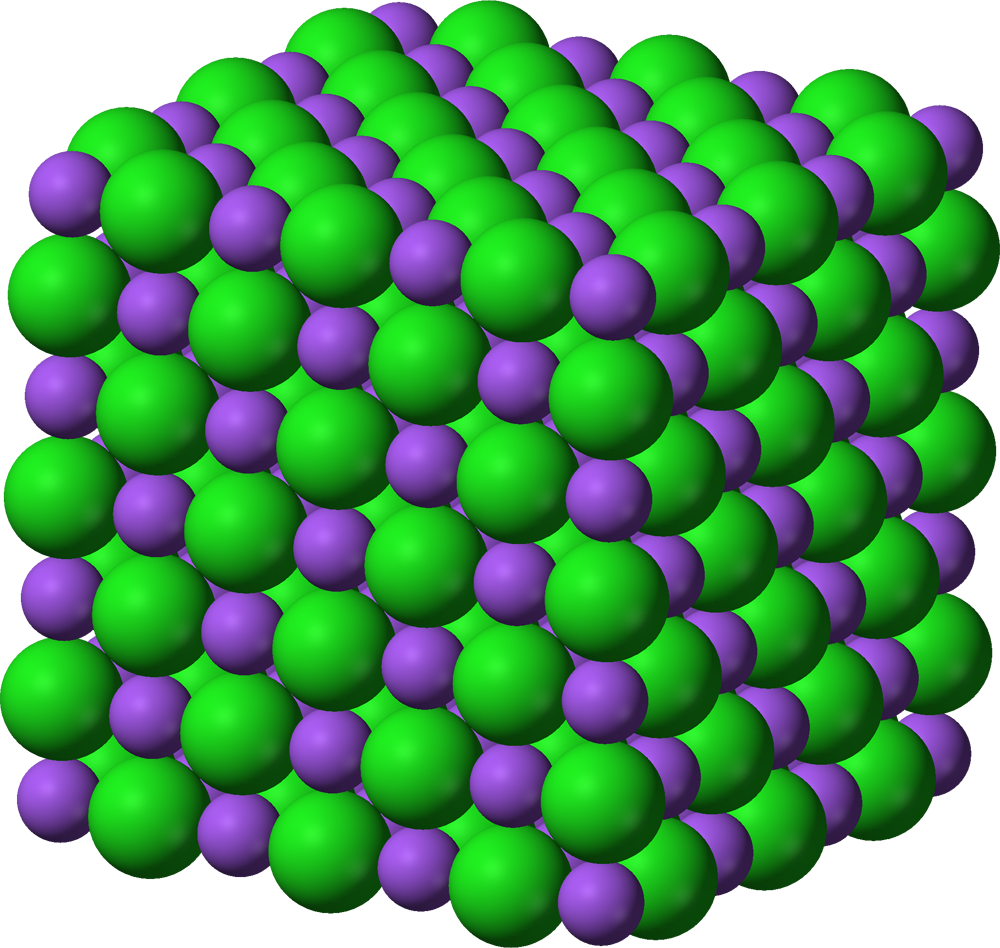
- sodium ions are in lattice, ions are not free to move, and cannot conduct charge, even though they are charged particles. the ions are not mobile.
- therefore, sodium chloride does not conduct electricity.
- however, sodium chloride solution does conduct electricity, because sodium chloride is disassociated into charged chloride and charged sodium.
- cathode and anion in beaker, cation go to cathode, chloride goes to anode.
- if ionic compound is soluble, then it is good electrolyte.
- the more soluble, the better electrolyte it is.
physical properties
- strong electrostatic forces of attraction (strong bonds).
- a network of ionic bonds (lattice), so a lot of bonds to break to separate.
- requires a lot of heat energy.
- usually has a high melting and boiling point
- requires a lot of heat energy.
- electrical conductivity
- not when solid, but when dissolved (always if they are soluble).
- are often soluble in water
- brittle
- ions can be displaced, and ions with the same charge could be located close to each other, which causes them to repel, and lead the structure to shatter.
lewis diagram
hey lois
- dot and cross diagram = lewis diagram!
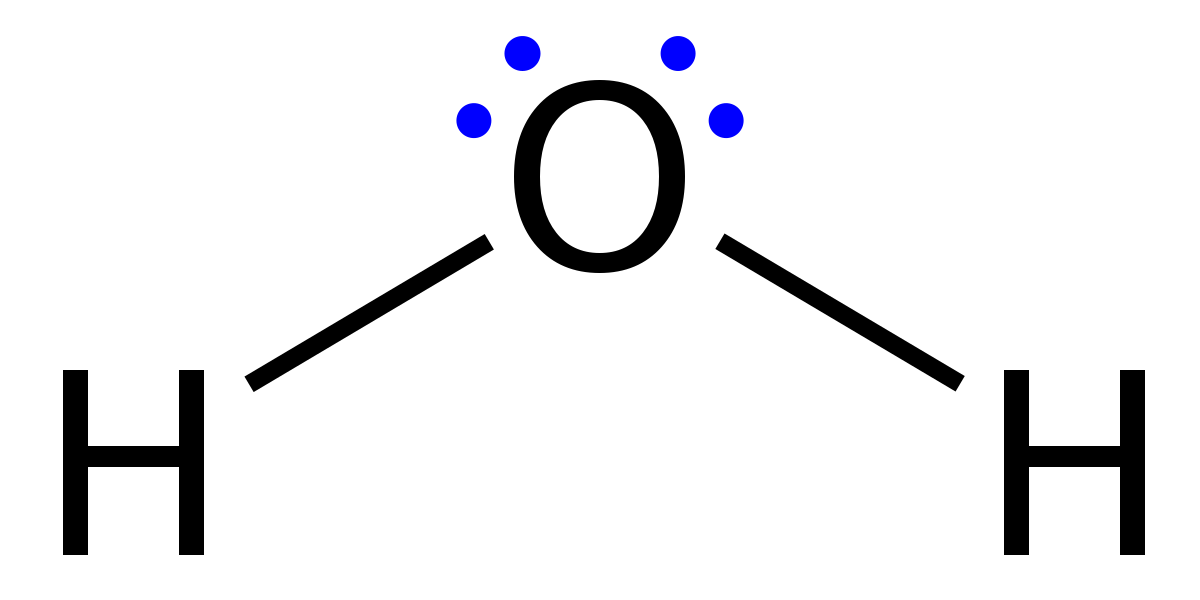
- lewis diagram
- dot-dot
- dot-cross
- show diagram of atom, and show what happens to it.
- show outer shell
- dot x dot, for dot x cross just use X instead of filled in dot :p