- In Ed’s opinion, the course should be structured in a way which: Equilibrium should be first -> Organic -> Industrial Processes & Redox
I believe Year 12 Redox can be taught in two hours - Edward Huynh
bold means important terms red highlight means “acceptable definitions for WACE” yellow highlight means the information is not 100% certain (either i misheard or something not fully covered in the course)
Redox Reactions
- Consider the Acid and Base theory, specifically the bronsted lowry theory:
- There exists Proton donors & Proton acceptors
- Redox reactions are similar, except there are electron donors & electron acceptors
- Some elements are said to be oxidised while others are said to be reduced
- Oxidise - losing/donating electrons.
- Reduce - gaining electrons.
- Oxidise + Reduce = Redox
- This can be remembered with the mnemonic OILRIG
- Oxidation
- Is
- Lose
- Reduction
- is
- Gain
- For something to oxidise and reduce, it must happen simultaneously.
- Generally transferred straightaway (in chemistry)
- Forced to oxidise by something else that reduces
- Reductant - something oxidises making something else reduces.
- Reductant is not something that reduces. energetically favourable for something else to reduce. mr beast giveaway electrons - reducing.
- Oxidant - something that reduces causing something else to reduce.
- similarly to oxidants and reductants - there are oxidising agent and reducing agent.
- anti-oxidants:
- prevents oxidants. oxidise to stop something else from oxidising. oxidise prefentially
- generally, if you want to do good in extended response, explore beyond what your teacher is talking about.
Oxidation Numbers
- oxidation occurs reaction-wise. quantify measures/reactions.
- most reactions are redox reactions - as they require the transfer of electrons.
- oxidation numbers - used to differentiate redox reactions and other reactions (e.g. acids and bases)
- a way to keep track of the extent to which an atom has gained or lost electrons.
- this thing has high tendency to oxidise etc etc.
- compounds / molecules all has oxidation numbers.
- elements exist in free state - oxygen as gas, metals as metals, oxidisation number of 0.
- monoatomic ions (Al3+) have an oxidation number equal to their charge. two half reactions work together combining for reaction to occur.
- Al goes from a state of 0 to 3+
- oxidation number increases -> becoming more oxidised.
- if oxidation number decreases -> becomes more reduced.
- even more ground rules. standard oxidisation numbers.
- hydrogen (most important): +1 except for specific case, when metal bonded to hydrogen (metal hydrides) (-1 instead of +1)
- oxygen: always -2, instead of peroxides (in -1)
- the sum of elements oxidation numbers = charge of polyatomic ion. phosphate is po43- -> oxidation number p = 4 x -2 + 3 = 5
- reduction potential
- going left -> right reducing
- right -> left oxidisation
- good at reducing E naut > 0, bad at oxidisation.
- spontaneous vs non-spontaneous reactions.
- this is electricity !! needs to be energy favourable. electrical potential of the redox reaction = electrical potential of the reduction + electrical potential of the oxidisation.
- Eo Cell = Eo Reduction + Eo Oxidisation
- positive = spontaneous
- spontaneous means happens immediately (in chemistry as soon as contact)
- NEGATIVE = NON SPONTANEOUS. (Does not happen immediately)
- electricity is weird. standard hydrogen half cell. arbitrarily made. half cell and measure the electricity output. putting the two blocks together electrically.
- what is a cell: redox reactions happen naturally. to visualise it, we make things called cells.
- use platinum in the cell thingy ?. because catalyst, conductive, not radioactive. (anything that uses a gas) - conductive but not reactive. electrolyte that corresponds with the metal - SOME METALS ARE INSOLUBLE TRICKY SO WE MAKE SURE OUR ELECTOLYTE IS SOLUBLE!
- spontaneous reactions produces voltage.
- batteries are like redox. essentially, you have two types of ??
- CLOCKWISE = SPONTANEOUS
- ANTICLOCKWISE = NON SPONTANEOUS
- standard conditions.
- consider stp 0 degres
- for redox, 25 degrees !!!
- standard concentration is 1mol/L
- these things affect reaction rate !!!!
- if reaction rate is higher for redox. there is a higher voltage! concentration temperature all affect reaction rate which affects voltage.
- more reaction rate = more charge transferred over time = more voltage
- batteries and corrosions.
- cells galvanic or electrolytic. positive or negative. how to read negative voltage :(
- we dont just read the reaction, we apply external voltage to the cell (just for the cell to happen), non spontaneous reactions require input (charge) in order to occur.
- galvanic cells. uses spontaneous reaction.
- galvanic cells convert chemical potential energy to electrical energy.
- voltage we are seeing on the voltmeter
- electrolytic uses non-spontaneous reaction.
- converts electrical energy to chemical potential energy.
- therefore, there are two different diagrams that we need to know.
- galvanic cells have crucial features we need to know.
- similarities:
- external circuit - WIRES (NOT water or ions !)
- anode (ALWAYS site of oxidation) CAN BE POSITIVE OR NEGATIVE, DEPENDS ON CELL TALK ABOUT POLARITY LATER
- cathode (site of reduction) can be positive or negative
- electrolytes are solutions (ed doesnt know why tho prob conduction)
- anions go to the anode, regardless of the galvanic or electrolytic.
- differences:
- we are moving electrons from one to another.
- (GALVANIC CELLS) salt bridge: provides a source of mobile ions that flow into half cells to maintain charge neutrality and complete the circuit
- we can also design a galvanic cell with a porous membrane instead of a salt bridge, since some atoms cannot pass through the battery to prevent spontaneous reactions.
- necessary to maintain the charge and complete the circuit. YOU CAN HAVE A SALT BRIDGE IN ELECTROLYTIC.
- voltmeter: reads the potential difference between the two things.
- POLARITY:
- IN GALVANIC CELLS: ANODES ARE NEGATIVE.
- flows from the negative end.
- CATHODE IS POSITIVE.
- in electrolytic cells: FLIPPED. ANODES ARE POSITIVE IN ELECTROLYTIC CELLS - because it is connected to the positive end. Cathodes are negative !!! OH em gee.
- DISCHARGES ELECTRICITY.
- IN GALVANIC CELLS: ANODES ARE NEGATIVE.
- similarities:
- REAL WORLD APPLICATIONS OF ELECTRICAL CHEMICAL CELLS.
- galvanic converts chemical potential energy to electrical energy.
- battery is not necessarily rechargeable. primary is non rechargeable, secondary is rechargeable.
- all primary cells are galvanic.
- DRY CELLS/LECLANCHE CELLS - NO WATER
- lok at battery diagram in lucarelli. (galvanic cell in industrial purposes)
- secondary cells are mi xed.
- discharging = galvanic, recharging is electrolytic.
- electrolytes cheap efficient and better.
- all primary cells are galvanic.
- comparison features for batteries
- energy density - how much energy it produces for how much it weighs, zinc is pretty low, lithium is very light, so it has high energy density.
- compare safety.
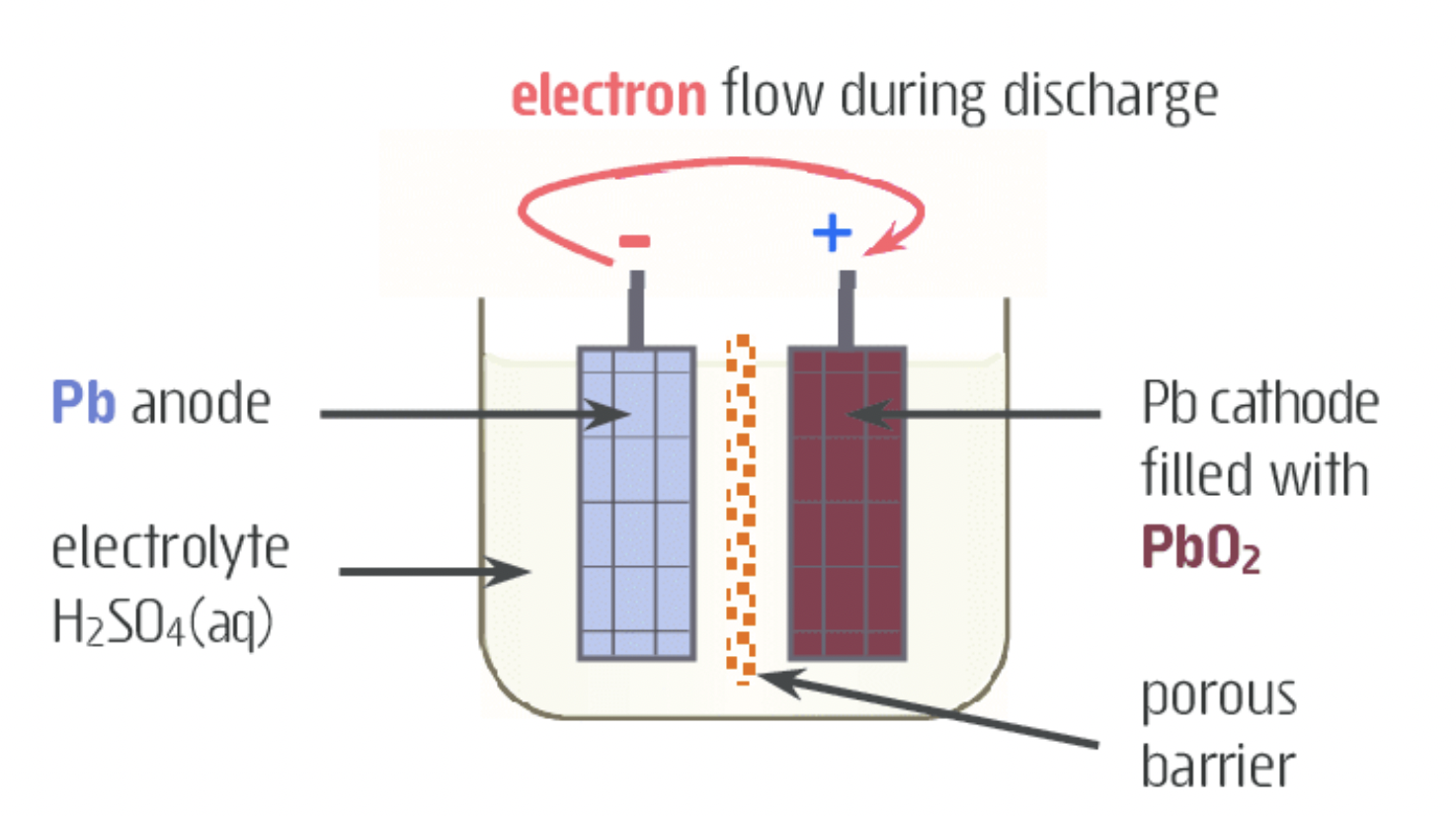
- lead acid accumulator - used in cars thumbs_up lasts for long.
- issue with secondary cells - it does not last forever. eventually batteries stop working.
- SECONDARY CELLS DEGRADE OVER TIME, WONT HAVE A PERFECT SECONDARY CELL!!
- fuel cells - literally what it sounds like, you fuel it in and it will last forever. consistent and usually used with less “dangerous” elements. we use light clean elements that produces clean things, which is the source of clean energy. (part of green energy)
- hydrogen and hydroxide makes water
- industry
- electroplating and electrorefining.
- ask teacher if 0 or = volts for electrorefining
- corrosion
- when we look at corrosion. oxidation of metals into salts. wet corrosion uses water, dry corrosion does not use water.
- why is corrosion bad. salt is brittle, so metal is not brittle. skyscraper not made my salt. turns part of the metal into salt (which is brittle).
- boat , brittle boat, water pull apart . hole in bolt. sink ANd die.
- what effects corrosion:
- concentration of oxygen in water
- heat
- rate of redox transfer
- amount of water.
- desiccate - absorbs all water.
- sealed: no oxygen - no water, oxidises the least. still undergoes dry corrosion, normal water
- be care of ions with plating some r catalysts - nickel maybe catalyses rate of corrosion (?)
- oxidises preferentially. strong reductants want to use.
- galvanising - coating iron in zinc.
- sacrificial anode - zinc, but instead of coating it, it acts as an electrical conductor. things will oxidise blah blah blah
- make this into a galvanic, battery - taking electrons, attach to metal, become cathode. This is called cathodic protection gaining electrons since ur forcing it to gain electrons, so no oxidisation. attach it to a power grid or something.