High Effort
Reversible Reactions
- Definition: A reaction in which the reactants become products (through a FORWARD reaction) and products become reactants (through a REVERSE reaction)
All Reactions Are Reversible. This is a trick question often set by WACE markers, every reaction is reversible, but the probability (P(reversible)) is not always more than 0.
- A reaction is only non-reversible if activation energy is too high.
- System: made of reactants and products, and reversible reaction is when reactants are constantly forming products and products are constantly forming reactants.
- Products - Reactants consistently interchange.
- Sometimes disruptions occur - more products are formed, or less reactants are formed.
- Imposed Changes: anything that disturbs dynamic equilibrium
ANYTHING AFTER HERE HAS NOT TURNED INTO ANKI YET!!!!!!!!!
- forward reaction: Reactants -> Products (low Ea for exothermic compared to Ea of endothermic)
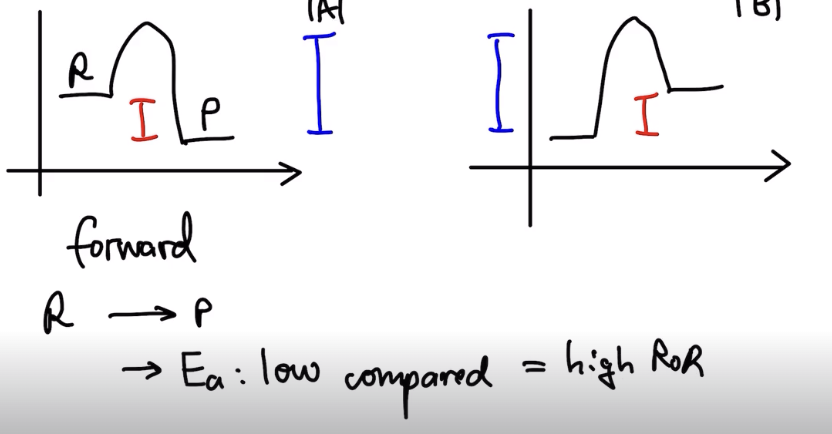
- Reverse Reaction -> Ae is high: lower rate as compared to forward.
- When a reaction starts, it is never equilibrium, it is net favoured, and F > R (5 vs 3)
- Evolution: Products increases: [P] increases: freq of col -> ROR of R increases
- Reactants decrease: [R] decrease: freq of col dec -> ROR of F decreases
- dynamic equilibrium occurs when the forward rate of reaction equal our reverse rate of reaction.
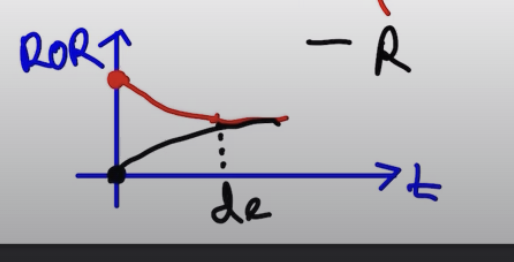
- when the shit collides and touches that is when dynamic equilibrium occurs.
- a reaction is reversible if Ea(forward) and Ea(reversible) are low-moderate
ALWAYS DRAW ENTHALPY DIAGRAM when explaining if something is reversible or not.
Always refer to activation energy for Forward AND Reverse.
Open and Closed Systems
- Open
- matter can be exchanged
- e.g. beaker with no lid
- Closed
- matter cannot be exchanged
- e.g. beaker with lid and insulated (wace only cares about lid)
- energy can be exchanged in both!
- we assume every reactants will form products and all products form reactants, but if the system is not closed, then some reactants and products are lost to surroundings, as they escape.
Concept of Dynamic Equilibrium
- Dynamic Equilibrium can only be established in Closed System.
- If matter is exchanged, you will lose reactants and you will lose products over time.
- Because otherwise lost P/R => no F/R reaction.
hidden marks in chem vv
Only occurs under two conditions (teachers are cancerous about this apparently) > - Only if the reaction system is closed. > - Only if the reaction can be seen as reversible.
- state of dynamic equilibrium is achieved if the forward and reverse rate of reaction is the same
- characteristics
- macroscopic properties (temp, colour, pressure[gases], odour) are constant. (they will never change)
- temp: assumed that no energy is being transferred. assumed its insulated, otherwise heat will flow in and out affecting dynamic eq.
- macroscopic properties (temp, colour, pressure[gases], odour) are constant. (they will never change)
- dynamic: looks (macroscopic) like nothing is changing: molecular level R <-> P
DYNAMIC EQUILIBRIUM GRAPHS
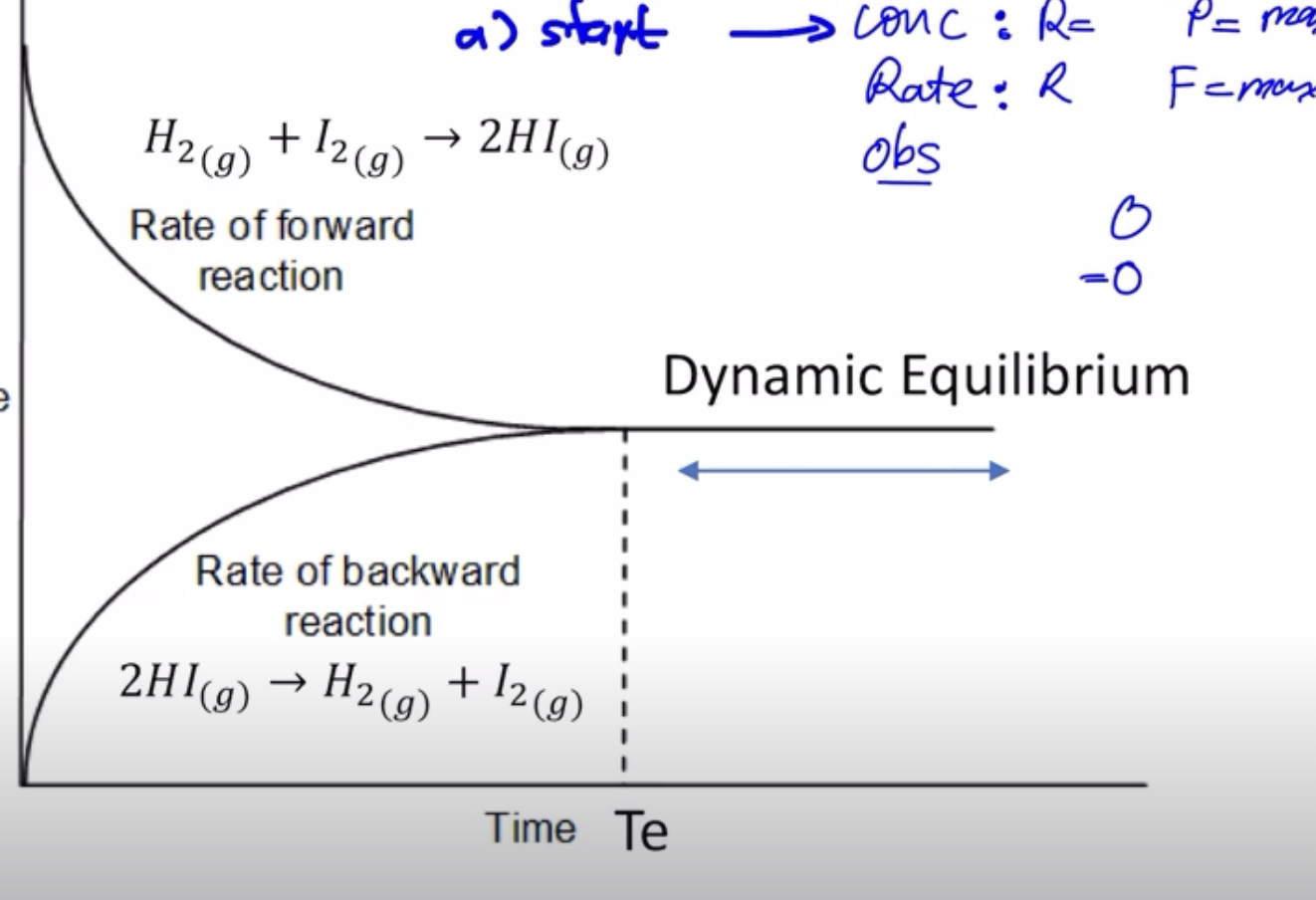
- start
- concentration: R max P=0
- rate: F = max R =0
- obs => red
- evolve
- conc: R DOWN (consumed) Prod UP (produced)
- Rate: Forward down, Reverse Reaction Rate Increases
- OBSERVATION: DO NOT SAY FADE, DO NOT SAY DISAPPEAR ONLY SAY DISCOLOURISE
- end: -> concentration: [R] = [P] CONCENTRATION IS SAME, BUT AMOUNT IS NOT THE SAME. If ratio is 1:1, [R] = [P]
- 1:2 [P] < [R]
- 2:1 [P] > [R]
- reactant and product concentration will remain constant
- rate: Forward = Reverse
- obs: constant pale red
- REMEMBER KEY WORDS AHH!H!H!H SCALING HAPPENS CLASS TO CLASS
- On graph, concentration vs time.
- change [R] = 1/2 change [P] if ratios exist STOIC RATIOS MATTER NOW.
- DE -> concentration dont change => never comment on ending concentration ONLY change
Exceptions
- h2o liquid vapour evaporation -> gas liquid
- rate [evaporation] = constant
- rate of evaporation depends on temperature
- there is no reliance on concentration (only: surface area)
- ROR evaporation: forward
- vapour starts at 0
- reaches forward
- vapour pressure = volume is a determinant of vapour pressure
- bunsen burner underneath it, increase temperature
- what is happening
- 2 differences
- physical
- forward or reverse reaction rate are never fixed in chemical equilibrium
- rate [evaporation] = constant
- solution equilibrium
- when you drop a solid into liquid,
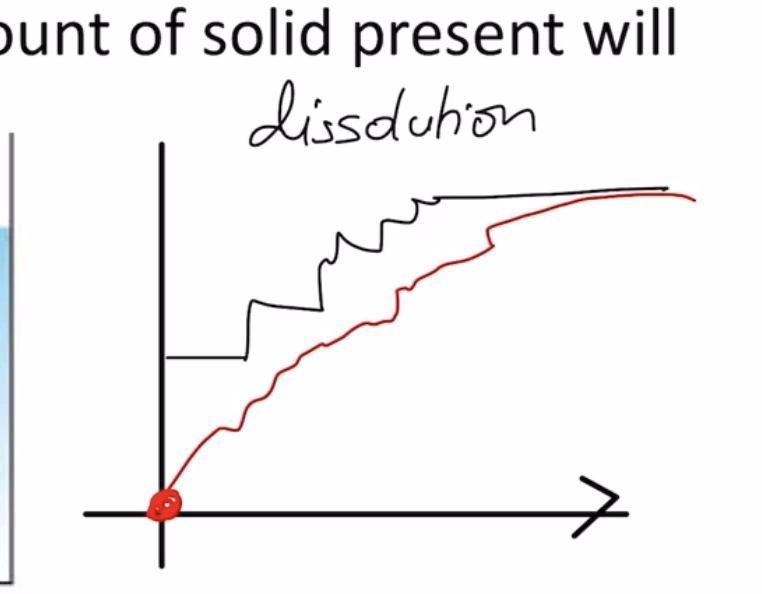
- they will never let u graph it cos its impossible
- dynamic equilibrium at the end!! even tho method is different.
- unsaturated => can increase
- super saturated: rate of dis is fixed
- saturated: is fixed
- the rate of dissolution and recrystallisation will eventually fall to 0
- subdivision phase occurs for a while, but once it is completely dissolved, the rate of dissolution will drop to 0
- when you drop a solid into liquid,
- equilibrium constant
- very important
- ratio of concentration(reactant):concentration(product)
- has to be 25 degrees
- 25 degrees if ?????
- at equilibrium [A], B, C, D
- H2 + I2 <-> 2Hi
- Kc = [HI]2/[H2][I2]
- p reaction is p
- stoic chemistry ratios as powers
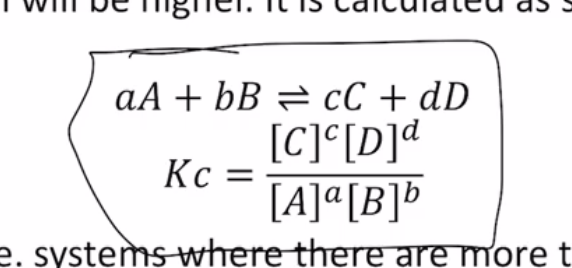
- Kc = 1: concentration of products = conc of R at end. favours neither reactant or product (when reaction is done, there will be more of neither reactants/rpdocuts)
- Kc > 1: conc products > conc reactants
- Kc < 1 conc reactants > conc products
- Kc determines acid strength =D
- uses of kc:
- acid base strenght
- electrolyte strength
- yield
- kc only works/is applicable in aqueous and gaseous reactants
- 2CaCO3 (s) + H2O (l) <-> Ca (s) + CO2 (g) + H2 (g)
- Kc = conc(CO2)conc(H2)
- does Kc ever change?
- temperature is the only factor that changes Kc, thus Kc is a fixed value
- if Kc = 10 = [CO2][H2]
- if Kc is a constant value, if you add more CO2, then all the extra will be converted to calcium carbonate.
- VERY IMPORTANT
- OCEAN ACID! 10 MARKS LOST!!
- WHEN YOU ANALYSE EQUILIBRIUM, WRITE KC! otherwise youd get trolled
- heterogeneous : s l q
- homogenous : all solid, all liquid
- if it is homogenous => include everything
Concept of Partial Pressure
- Partial Pressure is the pressure of gas molecules if it occupies the system by itself.
- If temp increases, pressure increases
- the partial pressure increases
- therefore if temp increases, partial pressure increases
- if temp increases, conc of aqueous products don’t change
Le Chatelier’s Principle
- never refer to as LCP
- if an imposed changed disrupts equilibrium, we need to be able to describe how equilibrium will be readjusted, ???
"According to Le Chatelier's Principle, the chemical system will re-establish equilibrium to partially counteract the imposed change."
- STATE WHICH DIRECTION EQ shifts and thus how the imposed change is partially counteracted.
EASY HACK
- step 1: identify change and make sure you can recognise it as an increase/decrease in some macroscopic property.
 REMEMBER THIS PUT THIS INTO ANKI =D
REMEMBER THIS PUT THIS INTO ANKI =D
- WE NEED TO DECREASE [CR2O72-] AND TO DO THAT, WE NEED TO FAVOUR THE RIGHT
REMEMBER YOUR ENDOTHERMIC AND EXOTHERMIC GRAPHS AND REACTIONS!!
- Kc never changes except with temperature
collision theory
- imposed change
- rate of forward partially dec and reverse inc
- forward reaction

- solution goes to NET INCREASE IN CRO72- (TYPO IN ABOVE!!)
Imposed Changes
imposed change 2: decreased reactant concentration
- 5 step process
- initial, forward, reverse reaction.
- talk about what happens over time, what happens when they converge
- what happens initially we decrease concentration of reaction, less mol less likelihood to collide.
- reverse rate of reaction
- if products not changed
- when you combine acid + base -> water is created, when you add water to the system 1. there is a miniscule system, 2. increase in water doesnt really matter (?cos ig the concentration added is miniscule)
- rate of reverse reaction is initially unaltered.
- net reaction is higher . initially we are at equilibrium, and ? decreases, the other stays the same.
- initially reverse rate of reaction > forward rate of reaction: reverse rate of reaction would partially increase/??
- since reverse reaction is higher, we will be consumed more products than produced.
- product [] decreases, reactant [] increase
- as we are increasing our reactant concentration, our forward rate of reaction is increasing over time
- as reverse rate is higher than forward, product conc decreases.
- trend happens until they converge, and at that point eq is reestablished
- hydroxide is removed from the system
- observations
- talk abt what happens with each colour
- orange <-> yellow
- solution becomes more orange
- net eq reaction
- if u remove ions or stuff, u are decreasing rate of eq.
- talk abt this =D
what will cnc vs time graph look like
- it doesnt matter where it goes, unless they tell you kc, it doesnt matter where they go
- adding Hcl, so hydroxide decreases
- if we r shifting to the left, dichromate & hydroxide should increase over time
- molar ratio (?): partial increase in conc OH-, net decrease
- if its above, u get marked down and shit fr
- equilibrium is partial counteracting of imposed change
partial pressure
- pressure if it existed in the container alone