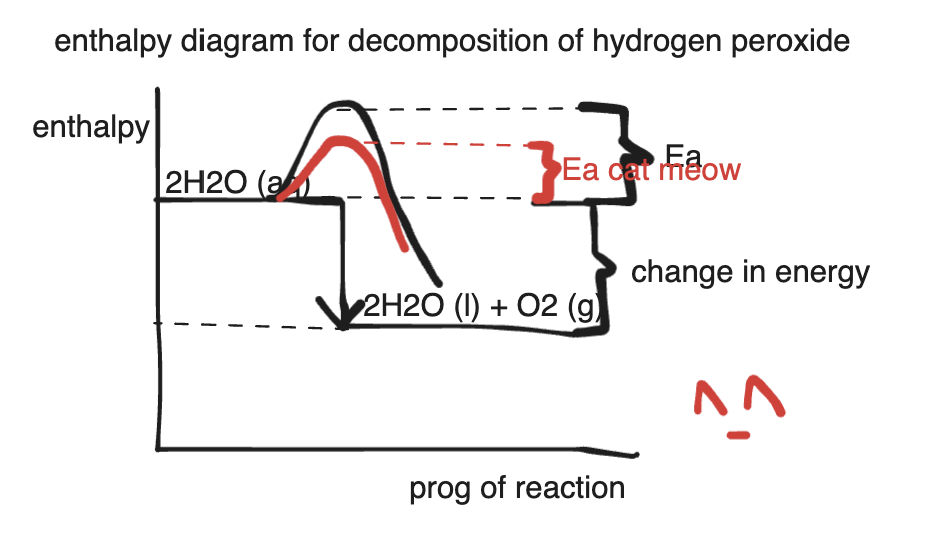
catalyst §
- decomposition of peroxide H2O2(aq)→2H2O(l)+O2(g)+heat
- process of this reaction happens as we speak, very very slowly (pilling holds bottle of hydrogen peroxide)
- to store it put in the fridge (lowers temperature, lowers rate of reaction -> converse is true, higher temperature, higher rate of reaction)
- you can buy hydrogen peroxide in chemist! (6%, we have 36% - which is corrosive
- )
- the activation energy for this reaction is quite high, as it reacts very slowly even in room temperature.