THIS COURSE OUTLINE IS COMPLETELY FOR PERSONAL USE. IT WILL PROVIDE NO VALUE AS ALL THE CONTENT IS SPECIFICALLY CATERED TOWARDS ME! I WILL NOT PROVIDE A TEMPLATE. MAKE IT YOURSELF.
Topic 1: REDOX
| Topic | Questions | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| 1 | 10/2/24 | |||||||
| 2 | 10/2/24 | |||||||
| 3 | ||||||||
| 4 | ||||||||
| 5 | ||||||||
| 6 | 10/2/24 | |||||||
| 7 | 10/2/24 | 11/2/24 | ||||||
| 8 | 11/2/24 | |||||||
| 9 | ||||||||
| 10 | 11/2/24 | |||||||
| 11 | 11/2/24 | |||||||
| 12 | 14/2/24 | |||||||
| 13 | 14/2/24 | |||||||
| 14 | 14/2/24 | |||||||
| 15 | 14/2/24 | |||||||
| 16 | 14/2/24 | |||||||
| 17 | ||||||||
| 18 | ||||||||
| 19 | 15/2/24 | |||||||
| 20 | 16/2/24 | |||||||
| 21 | 17/2/24 | |||||||
| 22 | 17/2/24 | |||||||
| 23 | 17/2/24 | |||||||
| 24 | 17/2/24 | |||||||
| 25 | 17/2/24 | |||||||
| 26 | ||||||||
| 27 | ||||||||
| 28 | ||||||||
| 29 | ||||||||
| 30 |
- 1. Describe oxidation as the process that involves the loss of electrons from a chemical species, and reduction as the process involving the gain of electrons by a chemical species.
- What is Oxidation? And what is Reduction?
- Oxidation is the loss of electrons. Reduction is the gain of electrons.
- In an Oxidation half-reaction, would the electrons be on the LEFT or RIGHT of the half-reaction equation?
- RIGHT
- The oxidant (oxidising-agent) is being ___, conversely the reductant (reducing-agent) is being ___
- REDUCED, OXIDISED
- What is a disproportionation reaction?
- Redox reaction where one species is oxidised and reduced
- only molecules of a polyvalent (3+ valency) element undergo disproportionation
- give example 2. Apply oxidation numbers to identify the species being oxidised and reduced in a redox reaction.
- What is oxidation number?
- way of keeping track extent atom has gained/lost electrons
- What does higher (more positive) oxidation number mean? (red/ox, e- lost/gained)
- Greater degree of oxidation
- Greater degree of electron loss
- What does the sum of the oxidation numbers of all atoms in the formula equal to?
- net charge shown on the formula.
- If oxidation number increases, is the reaction reduction or oxidation?
- oxidation 3. Write and balance half equations for oxidation and reduction in acidic conditions. 4. Write and balance redox equations in acidic conditions. 5. Provide observations for metal / metal ion and halogen / halide displacement reactions in aqueous solution. 6. Describe electrochemical cells as devices that allow for transformation between chemical potential and electrical energy.
- What are electrochemical cells?
- devices that allow transformation between chemical potential energy and electrical energy
- What are the types of electrochemical cells?
- Galvanic Cells
- Electrolytic Cells
- List 5 differences between Galvanic and Electrolytical cells?
- List 5 similarities
 7. Describe the key features of a galvanic cell using labelled diagrams.
7. Describe the key features of a galvanic cell using labelled diagrams.
- What is a galvanic cell? (3 Marks)
- A type of electrochemical cell that transforms chemical potential energy into electrical energy (1)
- In a spontaneous redox reaction that produces a voltage (1) (potential difference that drives an electric current. (1))
- What are the main features of a galvanic cell?
- Electrodes (cathode, anode)
- Provides pathway for the electrons to flow from the reductant in the oxidation half cell to the oxidant in the reduction half cell without direct contact
- Salt Bridge
- External Circuit
- Two Half Cells (reduction, oxidation)
- Voltmeter (optional)
- Electrolyte Solution
- What is the direction of electron flow in a Galvanic Cell
- Anode to Cathode
- Is Anode Positive/Negative
- Negative
- Why are the two half cells and electrodes separated? (3)
- If the reductant/oxidant came into physical contact, there would be an instant transfer of electrons and no electricity would be produced (1)
- Separating electrodes into 2 ½ cells allows for the reductant and oxidant to be separated and prevents them from coming into contact (1)
- Thus electrons are forces to flow through the external conductor and electricity is produced (1)
- Why is a salt bridge required in a galvanic cell?
- As e- move from the anode to cathode, if there was no salt bridge: the anode would become positively charged (as its lost electrons) and the cathode would become negatively charged (as it has gained electrons) (1)
- If this accumulation of charge is allowed, it opposes the motion of the electrons and thus overtime there is no further flow of electrons through the external circuit: i.e. the cell does not function (1)
- The salt bridge is required as it allows for ions to move between the electrons in the two half cells (anions to anode and cations to cathode) and thus prevents an accumulation of charge: i.e. completes the circuit and allows normal function of the cell (1)
- Why does the EMF produced decrease as the galvanic cell discharges?(3 Marks)
- As the cell discharged, the reactants are used up in the redox reaction and thus their concentration decreases (1)
- As concentration decreases, the rate of the redox reaction decreases and so the rate of electron transfer through the external circuit decreases: i.e. current decreases (1)
- As the voltage produced is proportional to the current, the voltage produced also decreases (1) 8. Describe and explain the role of the following in the operation of a galvanic cell:
- Electrodes (cathode, anode)
- a. Anode processes – using half equations
- site of oxidation
- b. Cathode processes – using half equations
- site of reduction
- c. Electrolyte
- solution of free/mobile ions that can flow and conduct and electric current
- It stores some reagents as well as allowing the flow of ions between the electrodes and the salt bridge: thus ensuring that there is no accumulation of charge and a complete electric circuit is formed
- solution of free/mobile ions that can flow and conduct and electric current
- d. Salt bridge and ion migration
- a non-reactive electrolyte solution that links the oxidation and reduction half cell. For example KNO3
- it provides a path through which ions flow between each ½ cell (anions to anode & cations to cathode) thus preventing an accumulation of charge and resulting in the completion of the circuit (1)
- a non-reactive electrolyte solution that links the oxidation and reduction half cell. For example KNO3
- e. Electron flow in external circuit.
- a conductor that joins the 2 electrodes
- provides a pathway for the electrons to flow from the reductant in the oxidation half cell to the oxidant in the reduction half cell without direct contact 9. Explain the use of a hydrogen half-cell as the standard for determining half-cell reduction potentials. 10. State that standard reduction potential (Eo) values are measured at solution concentrations of 1 mol L-1, temperature of 298 K, and gas pressure of 100 kPa.
- a conductor that joins the 2 electrodes
- what are SRP values measured at?
- STP
- 25degrees celsius
- 100kpa
- 1mol/L
- what are limitations of SRP?
- can only be used at 25deg, 1mol/L conc and pressures of 100kPa
- SRP ranking system: at altered conditions: rankings will change (i.e. reducing strength of species changes) making SRP useless
- does not give information about rate of chemical reaction.
- some reactions may be spontaneous according tot SRP, but may have larger activation energy: not occurring at “standard” conditions.
- only applied in aqueous solutions
- a lot of reactions in liquid, gaseous or even solid states are not included. 11. Use standard electrode potentials to calculate the electrical potential difference (Ecell) of an electrochemical cell.
- can only be used at 25deg, 1mol/L conc and pressures of 100kPa
- STP
- How do you calculate the Ecell of an electrochemical cell?
- SRP(reduced) + SOP(oxidised) 12. Describe spontaneous redox reactions as those reactions occurring in galvanic cells and fuel cells where the Ecell has a positive value.
- What is required for Spontaneous Reactions to occur?
- The Ecell needs a positive value. 13. Predict whether reactions will be spontaneous using standard reduction potentials.
- How do you predict spontaneity?
- Using SRP
- Figuring out Ecell, if Ecell > 0 (positive), it is spontaneous
- Ecell = SRP(oxidant) + SOP(reactant) 14. Write half equations and balanced equations for redox reactions occurring in galvanic cells.
- How do you write half equations
- refer to data table
- make sure reaction at the anode is oxidising, reaction at cathode is reducing. 15. Use standard reduction potentials to determine the relative strength of oxidising and reducing agents.
- what does higher SRP value mean (more positive)
- more likely to reduce
- contrary, higher SOP value means more likely to oxidise 16. Compare the voltages generated by electrochemical cells constructed from different materials, using Ecell values.
- what does higher Ecell mean?
- higher Ecell more voltage is generated, as Ecell = voltage 17. Conduct experimental work safely, competently and methodically to collect valid and reliable data, including the use of electrochemical cells. (SIS) 18. Organise and process data from experiments using galvanic cells, in order to compare reactivity of substances involved. (SIS) 19. Apply knowledge and understanding of electrochemical reactions in the context of corrosion of metals (dry corrosion and wet corrosion of iron) as an electrochemical process, and corrosion prevention by a range of techniques, including by exclusion of oxygen and/or water and through cathodic protection and sacrificial anodes.
- What are the two types of corrosion
- Dry Corrosion
- direct reaction with oxygen in air
- example of very reactive metal with air
- sodium
- oxide layer
- passivation
- the oxide layer formed may protect the metal from being further oxidised
- does iron have passivation?
- no, rust flakes away readily, exposing metal underneath
- passivation
- Wet corrosion
- presence of moisture accelerates rate of corrosion
- what are some other factors accelerating corrosion?
- pH
- lower pH -> more acidic, Fe2+ created faster as it also reacts with H+ ions
- presence of water
- temperature
- other pollutants
- such as salt or acid
- pH
- process
- iron ions reduction, hydroxide oxidation
- iron 2 hydroxide
- iron hydroxide oxidises to form iron 3 hydroxide
- iron hydroxide loses water to form hydrated iron oxide (RUST)
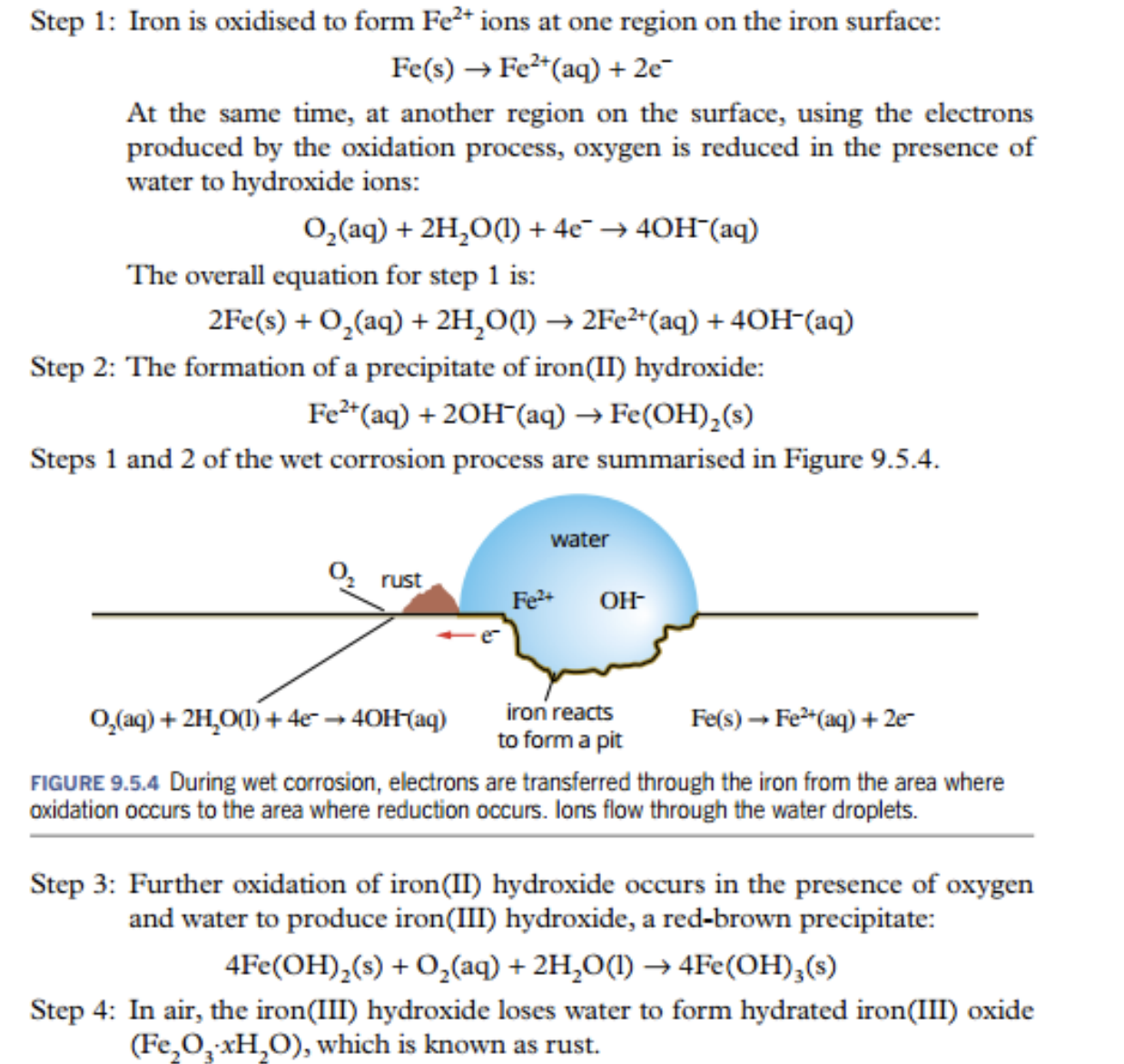
- Dry Corrosion
- prevention of corrosion
- surface protection
- paint, plastic
- prevents contacts with oxidant
- passivation
-
-
- cheap
-
-
-
- not permanent, eventually wears off, therefore you have to apply a new coat
- therefore if it scratches, not poggers
- doesnt cover porous metal -> incomplete
- not permanent, eventually wears off, therefore you have to apply a new coat
-
- paint, plastic
- electrochemical protection
- cathodic protection
- (ASSUME THEY MEAN VOLTAGE BASED. UNLESS STATED TO BE SACRIFICIAL ANODE)
- process
- applies an electric current on the target metal to force it to become a cathode (force it to reduce) by forcing electrons into the metal.
- connected to inert metal, buried in the ground
- lots of ions in the soil, causing it to react
-
-
- permanent (as long as you force electrons into it)
- green principles of chem (learn later)
-
-
-
- expensive => constant electricity
- maintenance => anode: change soil, cathode:
- if ur in a poor ass country => electricity isn’t constant, therefore it wouldn’t work without. it would immediately oxidised when the electric current is no longer applied.
-
- sacrificial anode 20. Apply knowledge and understanding of electrochemical reactions in the context and combustion reactions in both limited and excess oxygen environments.
- cathodic protection
- surface protection
- How do electrochemical cells operate, and what role do they play in various chemical processes?
- electrochemical cells transform between chemical potential energy and electrical energy.
- through the use of spontaneous and non-spontaneous redox reactions.
- Explain the environmental impact of electrochemical cells and processes, considering their operation and overall efficiency.
- dry cell (leclanche)
- cannot be reused, and has to go to landfill.
- however, there is little environmental impacts, only issue is that it is a little wasteful.
- hydrogen fuel cell
- pose no environmental impact (by itself) as reactants and products are oxygen gas, hydrogen gas and water
- however, production of hydrogen gas could cause negative environmental impacts such as greenhouse gas emissions.
- lead-acid cell
- leakage of sulphuric acid [[https://www.sciencedirect.com/science/article/pii/S1878029616001043?ref=pdf_download&fr=RR-9&rr=856654baea5a2ea0|(Study on the Environmental Risk Assessment of Lead-Acid Batteries)]]
- during process of transportation, production, processing, usage or storage.
- sulphuric acid is corrosive to living tissue, other metals and some stones.
- hygroscopic, readily absorbing water vapour from air
- if concentrated, strong dehydrating and oxidising properties.
- leakage of sulphuric acid [[https://www.sciencedirect.com/science/article/pii/S1878029616001043?ref=pdf_download&fr=RR-9&rr=856654baea5a2ea0|(Study on the Environmental Risk Assessment of Lead-Acid Batteries)]]
- dry cell (leclanche)
-
What are the key properties of substances involved in electrochemical reactions, and how do these properties influence reaction outcomes?
- Describe the combustion behaviour of substances in limited
- oxygen environments and its implications on reaction products.
- How does the combustion process differ when substances are exposed to excess oxygen, and what are the consequences for the environment?
- Explore the importance of understanding combustion reactions in the context of future environmental considerations. 21. Apply knowledge and understanding of galvanic cells in the context of batteries (primary: for example the Leclanché cell, and secondary cells: for example the lead‐acid accumulator and other secondary cells).
- Lenclanche Cell (Dry Cell)
- Primary Cell
- Electrochemical Galvanic Cell that is discharged in a spontaneous redox reaction to produce electrical energy.
- Reaction in dry cells cannot be reversed and regenerated once cell’s reagents are consumed.
- Anode: Zinc Solid oxidised to produce Zinc ions and electrons
- | SOP: 0.76V
- Cathode & cathode paste: Cathode paste consisting of and disassociated into NH4+ ions and Cl- where ammonium ions provide proton for reduction of MnO2
- porous fibre membrane:
- allows ion flow between cathode paste and anode, while preventing direct contact.
- Primary Cell
- Lead-Acid Accumulator 22. Apply knowledge and understanding of galvanic cells in the context of fuel cells for example, the hydrogen fuel cell.
- Hydrogen Fuel Cell 23. Describe electrolysis as a process in which electrical energy is used to produce chemical change. 24. Explain that electrolytic cells use an external electrical potential difference to provide the energy to allow a non-spontaneous reaction to occur. 25. Describe the key features of an electrolytic cell using labelled diagrams. 26. Describe and explain the role of the following in the operation of an electrolytic cell: a. Anode processes – using half equations b. Cathode processes – using half equations c. Ion flow in the electrolyte d. Electron flow in external circuit. 27. Write half equations and balanced equations for redox reactions occurring in electrolytic cells. 28. Explain why electrolysis of molten salts and aqueous solutions of salts may yield different products. 29. Describe and explain the differences between galvanic and electrolytic cells. 30. Describe and explain how electrolytic cells are used in a range of industrial situations, including metal plating (example Silver) and refining (example purification of copper).
- What is Oxidation? And what is Reduction?
Topic 2: EQUILIBRIUM
1. Use collision theory to explain and predict the effects changes in concentration, temperature, pressure, catalysts and surface area on the rate of reaction. 2. Describe chemical systems as either open (which allow matter and energy to be exchanged with the surroundings) or closed (which allow energy, but not matter, to be exchanged with the surroundings). 3. Explain observable changes in chemical and physical equilibrium systems at an atomic / molecular level. 4. Conduct experimental work safely, competently and methodically for the collection of valid and reliable data, including the effects of changes to equilibrium systems. (SIS) 5. Explain how over time, in a closed system, reversible physical and chemical changes may reach a state of dynamic equilibrium, with the relative concentrations of products and reactants defining the position of equilibrium. 6. Describe and explain the characteristics of a system in dynamic equilibrium, in terms of rates of reaction and macroscopic properties. 7. Explain the reversibility of chemical reactions in terms of the activation energies of the forward and reverse reactions. 8. Predict, using Le Châtelier’s principle, the impact of the following changes to a system initially at chemical equilibrium: a. changes in temperature b. changes in solution concentration c. changes in partial pressures of gases d. addition of a catalyst 9. Using collision theory, explain and predict how the changes listed above affect the rates of the forward and reverse reactions, and how this may lead to a shift in the position of equilibrium. 10. Predict the effect of changes in temperature on the position of equilibrium by considering the enthalpy changes of the forward and reverse reactions. 11. Use equilibrium constants to qualitatively predict the relative amounts of reactants and products at equilibrium. 12. Draw and interpret graphs of concentration against time and rate against time for equilibrium systems, including the effect of changes in concentration, partial pressure, total volume, total pressure and temperature. 13. Write equilibrium law expressions for homogeneous and heterogeneous systems. 14. Compare and explain the relative proportions of reactants and products at equilibrium by considering equilibrium constant expressions and values. 15. Explain how industrial processes such as the Haber process often involve a compromise of rate, equilibrium yield and economic considerations. 16. Evaluate the effect of carbon dioxide emissions on global temperatures, ocean pH and marine life. (SHE)
Topic 3: ACIDS & BASES
1. Define acids as substances that can act as proton (hydrogen ion) donors, and classify acids as monoprotic or polyprotic depending on the number of protons available for donation. 2. Define bases as proton acceptors. 3. Describe the ionisation of acids and the dissociation of bases with equations. 4. Explain the difference between the terms “strong” and “concentrated” when referring to solutions. 5. Identify strong acids (including HC, H2SO4 & HNO3) and strong bases (including NaOH, KOH & Ca(OH)2). 6. Describe the strength of acids and bases using the degree of ionisation at equilibrium in aqueous solution. 7. Identify weak acids (including H3PO4, CH3COOH & other organic acids) and weak bases (including NH3 & Na2CO3). 8. Conduct experimental work safely, competently and methodically to establish the properties of acidic and basic substances. (SIS) 9. Represent the strength of acids using the acidity constant, Ka, and use the value of Ka to describe the strength of an acid. 10. Identify conjugate acid – base pairs in equilibrium systems. 11. Explain how the Brønsted-Lowry model can be used to explain the relationship between acids and bases in equilibrium systems. 12. Use chemical equations to illustrate the transfer of protons between conjugate acid-base pairs. 13. Represent the hydrolysis of salts of weak acids and weak bases by using equations. 14. Use the Brønsted-Lowry model to explain the acidic, basic and neutral nature of salts derived from acids and bases. 15. Explain why the equivalence point of a neutralisation reaction is not always neutral (pH 7). 16. Describe the key features of a buffer solution. 17. Explain how buffer solutions resist changes in pH. 18. Describe the conditions that determine a solution’s buffering capacity. 19. Use collision theory and Le Châtelier’s Principle to predict how a buffer solution will respond to the addition of hydrogen ions or hydroxide ions. 20. Describe the self-ionisation of water using an equation, and explain why water is a weak electrolyte. 21. Use the ionic product of water, Kw = [H+][OH-] = 1.0 x 10-14 at 25oC, to quantify the self-ionisation of water. 22. Explain why the value of Kw is temperature dependent, and why neutral solutions can have a pH other than 7. 23. Use Kw to calculate the concentration of hydrogen ions or hydroxide ions in solutions of strong acids and strong bases. 24. Describe the relationship between hydrogen ion concentration and pH using the pH scale (an inverse logarithmic scale). 25. Calculate the pH of a solution from the hydrogen ion concentration using pH = - log10[H+], and the hydrogen ion concentration from the pH using [H+] = 10-pH. 26. Use pH or [H+] to calculate the hydroxide ion concentration of a solution. 27. Calculate pH, [H+] and [OH-] when solutions of strong acids and bases are mixed. 28. Recognise that pH and Ka values are inversely related for equimolar solutions of weak acids. 29. Describe acid-base indicators as weak acids or bases, which have different structures and colours in their protonated and deprotonated forms. 30. Explain how the change in pH of a solution causes acid-base indicators to change colour. 31. Explain the difference between the equivalence point of a reaction and the end point of an indicator. 32. Justify the selection of an indicator for an acid-base titration on the basis of the pH of the reaction’s equivalence point and the indicator’s end point. 33. Draw and interpret pH curves for neutralisation reactions between strong and weak acids and bases. 34. Perform titration calculations to establish the concentration of an acidic or basic solution. 35. Explain the reasons why back titrations are performed. 36. Perform back titration calculations to establish the concentration of an acidic or basic solution. 37. Communicate effectively using appropriate language, nomenclature and format in a scientific report. (SIS) VOLUMETRIC ANALYSIS 38. Describe the principles underlying volumetric analysis. 39. Select an appropriate indicator for an acid-base titration. 40. Describe how to create an accurate primary standard solution. 41. Explain the reasoning behind the choice of substance for a primary standard solution. 42. Calculate the concentration of a solution using titration data. 43. Use outcomes from titration calculations to reflect on purity and composition of substances. 44. Describe what systematic and random errors are and explain how they affect the results of a titration. 45. Calculate percentage error on a measurement. 46. Describe how to minimise experimental error (systematic and random) in titration. 47. Evaluate the effects of experimental errors on the outcomes of titration calculations.
SEMESTER 1 EXAMS!!
- Redox, Equilibrium, Acids & Bases. 48. Demonstrate competence using volumetric equipment. (SIS) 49. Demonstrate competence in collecting accurate and precise titration data. (SIS)
Topic 4: ORGANIC
1. Revision: Write IUPAC names, molecular, condensed and structural formulae of the following, for up to 8 carbon atoms. a. Alkanes – straight and branched chain b. Cycloalkanes c. Alkenes – straight and branched chain d. Cycloalkenes e. Alkynes – straight and branched chain f. Alkyl groups g. Haloalkanes 2. Revision: Identify and describe aromatic compounds, as substances containing a benzene ring. 3. Revision: Describe isomers as compounds with the same molecular formula but different structures. 4. Revision: Draw and name isomers, specifically chain isomers, position isomers and geometric (cis-trans) isomers. 5. Describe functional groups as groups of atoms or bonds that are responsible for the characteristic chemical properties of a molecule or homologous series of molecules. 6. Identify the functional groups for alkenes, alcohols, aldehydes, ketones, carboxylic acids, esters, amines and amides. 7. Use IUPAC nomenclature to write names for organic species with a parent chain of up to 8 carbon atoms and simple branching, and one of the following functional groups: alkenes, alcohols, aldehydes, ketones, carboxylic acids, esters, amines and amides. 8. Draw structural and condensed formulae for organic species with a parent chain of up to 8 carbon atoms and simple branching, and one of the functional groups listed above. 9. Explain that functional groups undergo specific reactions which can be used to identify the functional group present, including: a. Addition reactions of alkenes b. Redox reactions of alcohols c. Acid-base reactions of carboxylic acids. 10. Use observations from reactions with bromine or iodine solutions to identify the presence of a carbon-carbon double bond in an alkene. 11. Describe the following reactions of alcohols using balanced equations, and provide expected observations: a. Complete combustion b. Oxidation of primary and secondary alcohols by oxidising agents such as acidified MnO4- or Cr2O72-. 12. Describe and explain the different outcomes for oxidation of primary, secondary and tertiary alcohols. 13. Describe the sequential oxidation of primary alcohols to produce aldehydes and carboxylic acids. 14. Use observations from reactions with acidified oxidising agents to identify the type of alcohol present. 15. Describe the following reactions of carboxylic acids using balanced equations, and provide expected observations: a. Acid-base reactions b. Acid catalysed condensation reactions with alcohols to produce esters. 16. Use observations from reactions to identify the presence of a carboxylic acid. 17. Describe the intermolecular bonding in a substance, and explain how the functional groups present bring about this bonding. 18. Explain the characteristic physical properties of organic compounds, including boiling point and solubility (in water and in organic solvents), in terms of intermolecular forces (dispersion, dipole-dipole and hydrogen bonding). 19. Determine the empirical and molecular formulae of organic substances from analytical data. 20. Use empirical and molecular formulae, along with data from chemical reactions, to suggest the structure of an organic compound. 21. Describe and write equations for addition polymerisation reactions, including the production of polyethene and polytetrafluoroethene. 22. Describe and write equations for condensation polymerisation reactions, including the production of polyesters and polyamides for example for example, nylon and polyethylene terephthalate. 23. Predict the structure of addition and condensation polymers from its monomer(s), and vice versa. 24. Describe the varied structures of different plastics due to its characteristics, including crosslinking, chain length, and intermolecular forces which leads to a range of distinct properties and consequent uses 25. Give some uses of common polymers including polyethene, polytetrafluoroethene, nylon and polyethylene terephthalate, and explain how their properties make them suitable for those uses. 26. Recognise and draw the generalised structure for α-amino acids. 27. Describe the characteristic properties of α-amino acids, including the ability to form zwitterions. 28. Explain why the pH of solutions of α-amino acids is not always pH 7. 29. Describe how α-amino acids undergo condensation reactions to form polypeptides and proteins, by the formation of amide (peptide) bonds. 30. Describe the primary structure of a protein as its sequence of α-amino acids. 31. Describe and draw the common secondary structures in proteins (α helices and β sheets), and explain how they result from the hydrogen bonding of amide groups on the backbone of the protein chain. 32. Describe the tertiary structure of a protein as the overall 3D shape, and explain how interactions between the side chains of α-amino acids holds it together. 33. Describe and draw the interactions between parts of the protein chain that contribute to its tertiary structure, including disulfide bonds, ionic bonds, hydrogen bonding, dipole-dipole interactions and dispersion forces. 34. Describe the role of the Protein Data Bank. 35. Communicate to specific audiences and for specific purposes using appropriate language, nomenclature, genres and modes, including scientific reports. (SIS )
Topic 5: SYNTHESIS
1. Explain that chemical synthesis to form products with specific properties may involve the construction of reaction sequences with more than one chemical reaction. 2. Explain why particular reactions, reagents and reaction conditions may be selected, in order to optimise the rate and yield of production. 3. Evaluate the choices of reaction conditions in order to optimise rate and yield in the following processes: a. Production of ammonia (Haber process) b. Production of sulfuric acid (Contact process) c. Production of biodiesel i. base-catalysed method ii. lipase-catalysed method. 4. Calculate quantities of reactants and products in a chemical reaction, including limiting reagents, using stoichiometric ratios. 5. Calculate the percentage yield of a chemical reaction and use this to comment on the overall effectiveness of the reaction. 6. Use percentage yield values for individual stages of a multi-stage synthesis to calculate the overall yield of the process. 7. Describe how enzymes can act as biological catalysts and explain the benefits and drawbacks to using enzymes in industrial synthesis. 8. Explain why enzymes work best within specific ranges of conditions, including temperature and pH. 9. Compare and contrast the production of ethanol via fermentation and hydrolysis of ethene, and explain the advantages and disadvantages of each method. 10. Describe the synthetic route for the production of ethyl ethanoate, linking to the production of ethanol and ethanoic acid, and their subsequent esterification reaction. 11. Describe how biodiesel is produced from fats and oils, via base-catalysed and lipase-catalysed processes, and evaluate the advantages and disadvantages of each method. 12. Describe how soap is produced by base hydrolysis (saponification) of fats. 13. Describe the structure of soaps as a non-polar hydrocarbon chain and a carboxylate group; and the structure of the anionic detergents derived from dodecylbenzene as a non-polar hydrocarbon chain and a sulfonate group. 14. Explain the cleaning action of soaps, using condensed structures of soap particles, and discussion of intermolecular bonds formed by the hydrophilic and hydrophobic sections. 15. Use balanced equations to describe the reactions of soaps with calcium and magnesium ions. 16. Describe the structure of detergents using a diagram. 17. Explain why detergents are preferable to soaps in some circumstances, using knowledge of solubility of the salts produced. 18. Evaluate the sustainability, resource requirements, economics and environmental impact of synthesis methods, including the production of ethanol and biodiesel. 19. Identify, research, construct and refine questions for investigation; propose hypotheses; and predict possible outcomes. (SIS) 20. Design investigations, including the procedure(s) to be followed, the materials required, and the type and amount of primary and/or secondary data to be collected; conduct risk assessments. (SIS) 21. Conduct investigations safely, competently and methodically for the collection of valid and reliable data. (SIS)
