corrosion
- corrosion is a redox reaction
dry corrosion
- direct reaction with oxygen in air to form metal oxide is known as dry corrosion.
- sodium is very reactive, it must be stored under oil to prevent contact with oxygen and corrosion.
- some dry corrosion, like with aluminium forms tough, impervious coating of aluminium oxide , protecting metal from further contact with oxygen (this is called passivation).
- iron on the other hand, is less reactive and when it does corrode, the coating it forms flakes, so it is unable to protect the iron underneath.
- things to note
- active corrosion: porous oxide layers may form, and the corrosion will continue deep into metal.
- sensitive to temperature: temp increases -> ROR increases
- 3 types
- oxidative corrosion
- liquid metal corrosion
- corrosion by other gases
- (so2, co2) gases react with exposed metal oxidising it.
- remember hydrogen sulfide on silver
- (so2, co2) gases react with exposed metal oxidising it.
wet corrosion
- electro-chemical
-
- metal
-
- gas
- liquid -> h2o
- Fe + O2 + H2O is common
- draw diagram lots of times to get it
- basic iron corrosion process
- iron solid oxidises and h2o and o2 reduces to form iron hydroxide
- this is not rust however, because this is not the colour of rust.
- electrons are transferred through iron solid to second region where o2 is reduced in the presence of water to hydroxide ions
- then forms fe(oh)3
- further oxidised in the presence of o2 and h2o to produce iron(iii)hydroxide: red-brown precipitate
- then forms fe2o3
- flaky; porous rust, exposes iron underneath to further corrosion.
- iron solid oxidises and h2o and o2 reduces to form iron hydroxide
- rate of rust AFFECT
- humidity => h2o in air UP => rate of rust UP
- temp => rate of rust UP => freq of col UP, % of succ UP
- prevention:
- DOWN temp => prevents
- in industry , materials corrode over time
- DOWN temp in factory
- contact process
- SO2+O2 -><- SO3
corrosion prevention
1 surface protection
- painting
- preventing contact w/oxidants
- drawbacks
- paint fades/flakes away => x permanent
- paint doesnt cover porous metal => incomplete
2 cathodic protection
- voltage based THINK OF THIS AS CATHODIC PROTECTION
- definition: target metal (save): force it to become a cathode (site of reduction)
- we force electrons into the site of reduction (attach negative side of battery)
- the inert metal becomes anode
- inert metal is submerged under usually water/soil (tons of metal ions) causing it to react
- forced our target metal: cathode therefore no oxd can occur = no corrosion
- drawbacks
- expensive: constant electricity => permanent
- maintenance:
- anode: change soil
- anode soil needs to be changed because the ions gets depleted n stuff 9?
- cathode: scratch (?)
- anode: change soil
- CONTEXT BASED: electricity => wont work without
- benefits
- permanent
- green principles of chem
- utilising sacrificial anode (plating)
- more R => metal reacts as an anode
- preferentially/sacrificially oxidised
- this forces our metal to be a cathode =D
- drawbacks:
- constantly renew: not permanent, more R metal has to be replaced
- if target metal comes into contact with less R metal, then the target metal will be forced to oxidise. therefore, cant be used where metals/ions are present
- benefits
- cheap
- whenever cathodic protection (voltage based) cannot be used 3 plating protection
- more R => metal reacts as an anode
- plate (coat)
- inert: less reactive
- less reactive coating
- less R oxidises instead of target metal-> eventually forms oxide layer: forms protective layer
- prevents contact between gas/o2
- drawbacks (scratching problem)
- why corrosion rate increases as scratches
- oxidants can now contact Fe
- fe will directly oxidise instead of cadmium: higher SOP (corrosion)
- cd is in contact with o2, thus acting as a site of Red: cd increases surface area of the metal, e- released can flow through Fe and Cd
- cause rate of ox increase, which increases rate of reaction.
- why corrosion rate increases as scratches
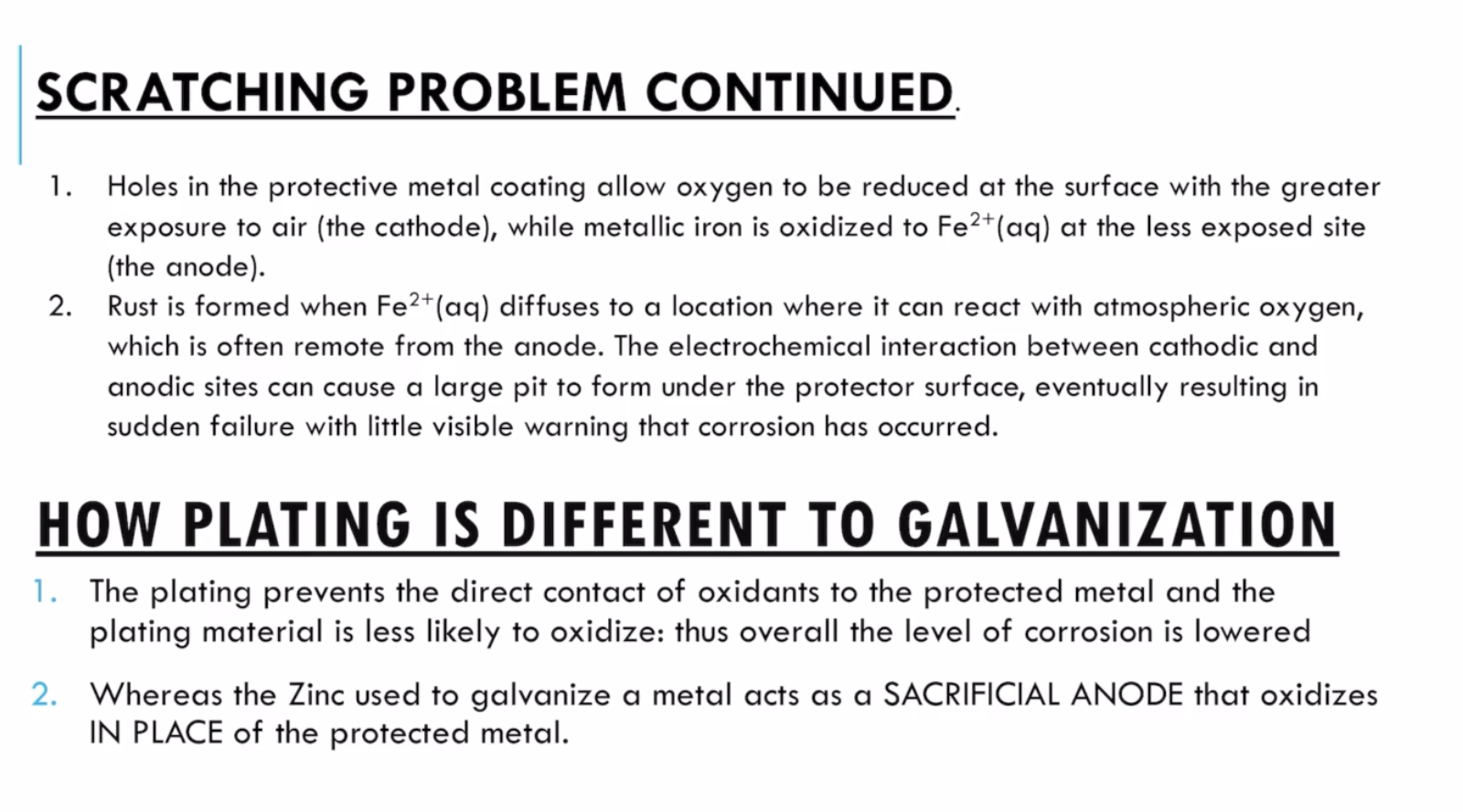
- galvanisation: more reactive
- more R: higher SOP
- this more R metal reacts instead of the metal you want.
- this more reactive metal is called sacrificial anode.
- galvanisation is electroplating of zinc onto target metal
- coating target metal = more R metal.
- drawbacks
- only protects metal less R than zinc
- not permanent: coat it again
- inert: less reactive