-
electroplating

-
electrorefining
- extracting pure metal (mixture) electrolyte
-
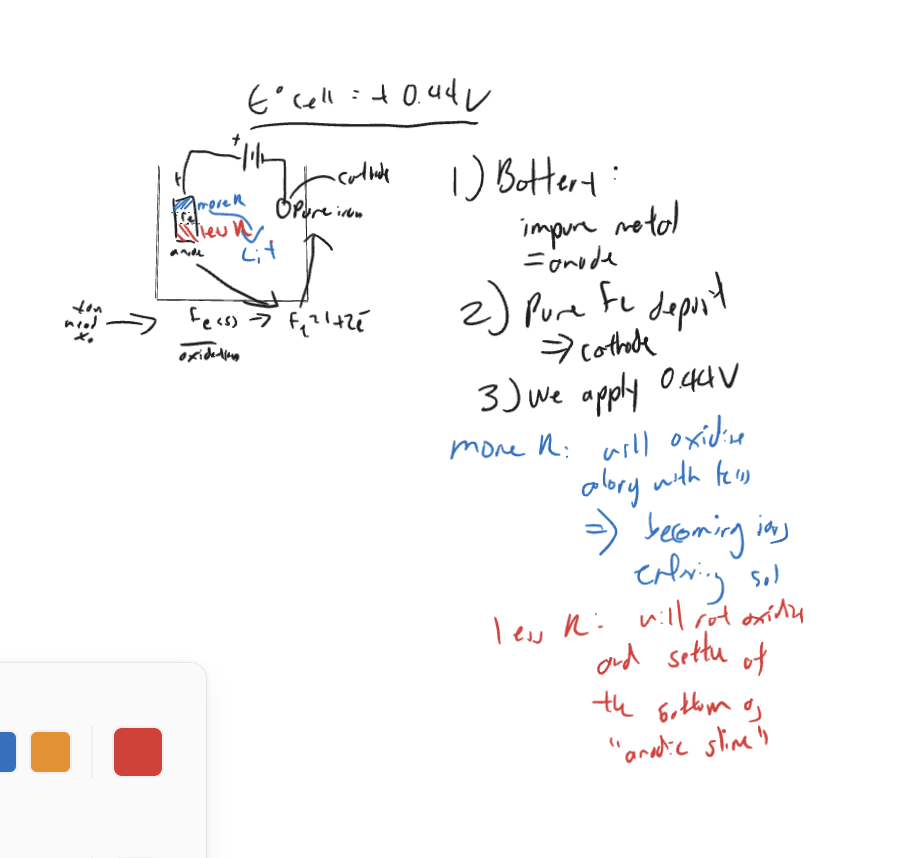
-
- at the cathode: SRP: Li+, Na+, Fe2+
-
metals that are more R(eactive) produce cations with lower SRP values
-
as E cell = SRP of Fe2+, anything w/ lower SRP: does not reduce!
-
anything more reactive than iron will just remain in solution.
-
separation 1: anode (less reactive)
-
separation 2: cathode (more reactive)
-
3 types of metal in the thingy
- the metal itself
- metal more reactive (higher sop)
- metal less reactive (lower sop)