energy & change of state §
- if water is heated -> temperatures will rise.
- if enough energy is transferred to the water, the water will boil.
- latent energy is the energy released or absorbed during a change of state (e.g. when water is boiled, when water is frozen etc etc)
- latent means hidden/unseen. latent heat is present, but we just do not see it.
- when a substance changes state, temperature remains constant
- image for anki flashcards :grin:
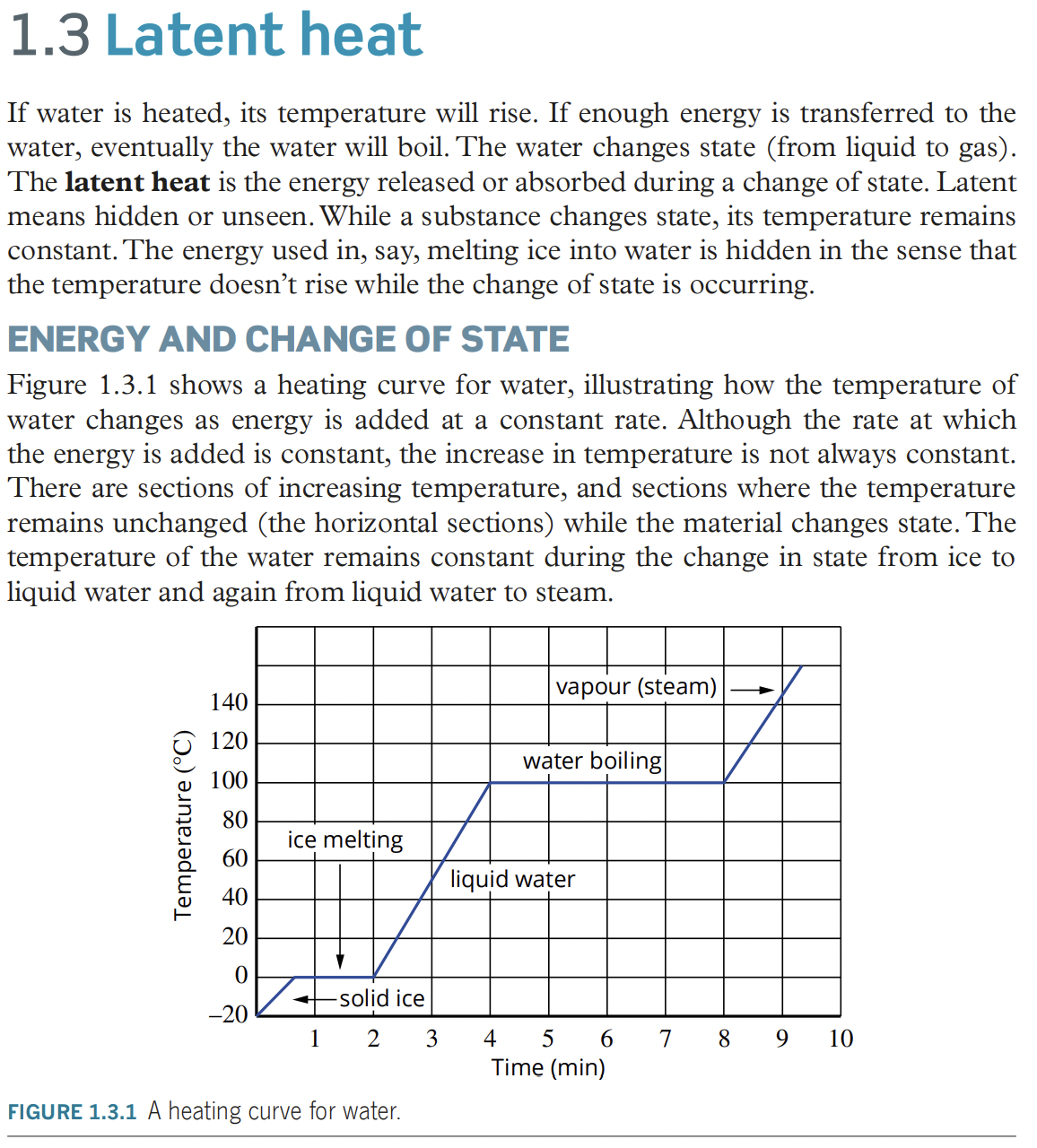
- temperature is added at a constant rate, but there are sections where temperature remains unchanged while the material change states.
latent heat §
- the energy needed to change the state of a substance is called latent heat
- latent heat is calculated using the equation: Q=mL
- where Q is the heat energy transferred in joules (J)
- m is the mass in kilograms (kg)
- L is the latent heat (Jkg^-1)
latent heat of fusion (melting) §
- as thermal energy is transferred to a solid, temperature of solid increases.
- particles within solid gain internal energy (as mostly kinetic energy and some potential energy) and speed of vibration increases
- internal energy: sum of potential and kinetic energy in substance.
- at the point solid begins to melt, the particles move further apart, reducing the strength of the bonds holding them in place.
- instead of increasing temperature, the extra energy increases the potential energy of the particles, reducing the interparticle or intermolecular forces.
- tldr: no change in temeprature occurs, as all the extra energy supplied is used in reducing forces between particles.
- amount required to melt solid = potential energy released when liquid reforms into a solid. it is termed latent heat of fusion
- for a given substance:
- heat energy transferred = mass of substance x specific latent heat of fusion
- Q=mLfusion
- where Q is the heat energy transferred in joules (J)
- m is the mass in kilograms (kg)
- Lfusion is the latent heat of fusion in Jkg−1
- 80 times as much energy to turn 1kg of ice into water (no temp change), as it does to raise temperature of 1kg of water by 1degC.
- it takes a lot more energy to overcome large intermolecular forces within ice than it does to simply add kinetic energy in raising the temperature.
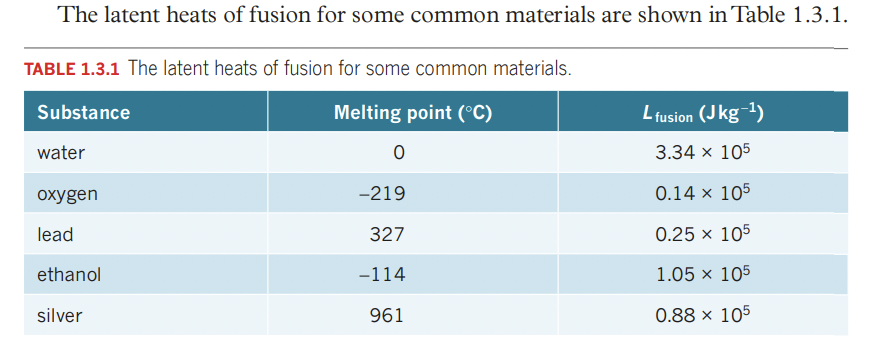
- latent heat vaporisation Q=mLvapour (boiling)
- average kinetic energy decrease as higher kinetic energy particles will have equipped the liquid.
- dependent on the volatility of the liquid, the surface area, the temperature, the humidity, and the air movement.

